J Korean Med Sci.
2007 Apr;22(2):262-269. 10.3346/jkms.2007.22.2.262.
Modified Acellularization for Successful Vascular Xenotransplantation
- Affiliations
-
- 1Department of Thoracic and Cardiovascular Surgery, Ansan Hospital, Korea University, Ansan, Korea.
- 2Department of Thoracic and Cardiovascular Surgery, Guro Hospital, Korea University, 80 Guro-dong, Guro-gu, Seoul, Korea. sohnys@korea.ac.kr
- 3Department of Pathology, Cheonan Hospital, Soonchunhyang University, Cheonan, Korea.
- KMID: 1713185
- DOI: http://doi.org/10.3346/jkms.2007.22.2.262
Abstract
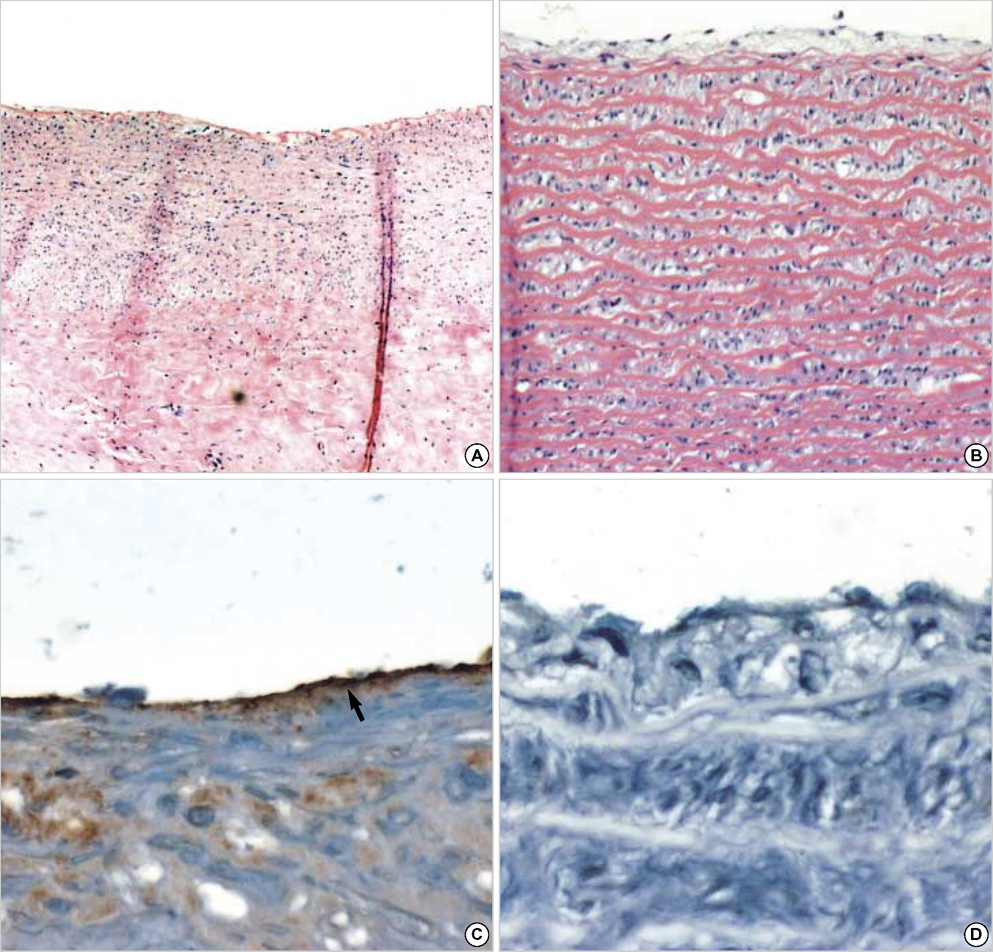
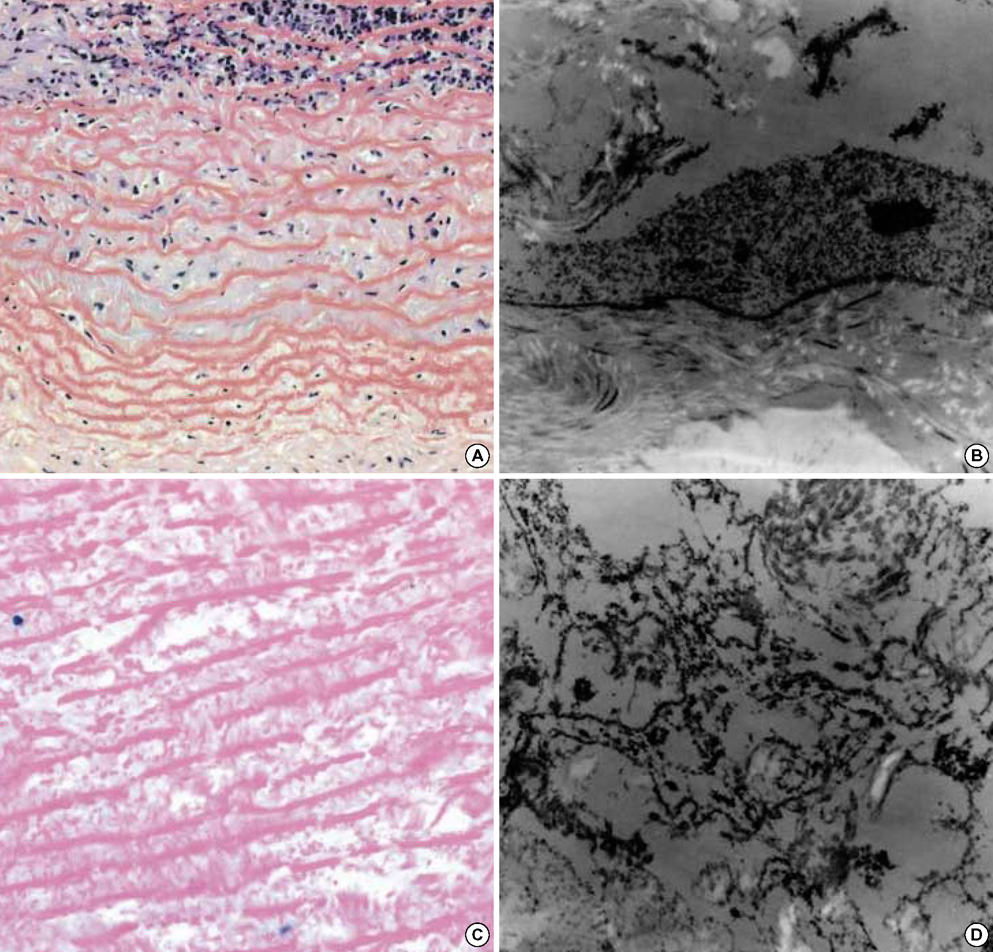
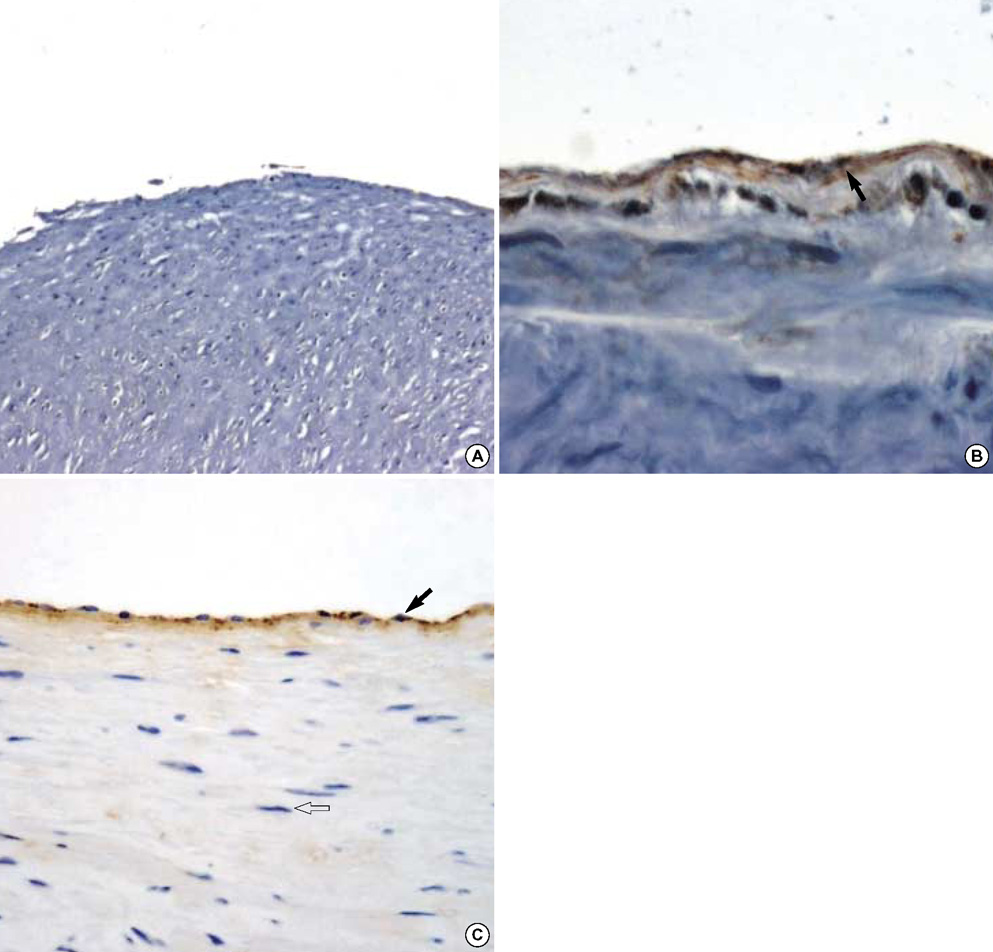
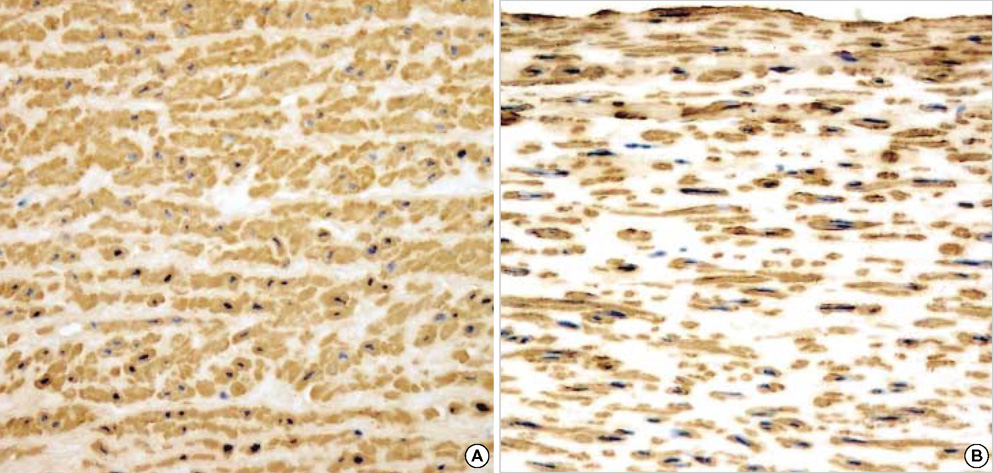
- The purpose of this study was to estimate the possibilities of an acellular matrix using a modified acellularization protocol, which circumvents immunological, microbiological, and physiological barriers. We treated porcine subclavian arteries with various reagents to construct acellular grafts. Afterwards, these grafts were interposed in a mongrel dogs' abdominal aorta. Six dogs underwent interposition with fresh porcine grafts (control group), and seven had interposed acellular grafts (acellular group). The control and acellular group dogs were sacrificed at 1, 3, 5 (n=2 in each group) and 12 months (n=1 in acellular group) after the operation. Histopathological examinations were then performed, to assess the degree to which re-endothelialization, inflammation, thrombus formation, and calcification occurred. The entire acellular group, but none of the control group, exhibited re-endothelialization. The degrees to which inflammation, thrombosis, and calcification occurred were found to be lower in the acellular group. We also discovered many smooth muscle cells in the medial layer of the xenograft that had been implanted in the dog sacrificed 12 months after the operation. These results suggest that the construction of xenografts using our modified acellularization protocol may offer acceptable outcomes as a vascular xenograft.
MeSH Terms
Figure
Reference
-
1. Kim HM, Song YJ, Sohn KH. A study on the manufacture of the artificial cardial tissue valve. Korean J Thorac Cardiovasc Surg. 1979. 12:383–394.2. Lee JY, Oh SM, Han JI, Kim MH, Sohn KY, Kim HM, Lee IS, Kim KT, Kim YH, Lim CY, Sohn YS. Development and animal tests of artificial heart valve. Korean J Thorac Cardiovasc Surg. 1987. 20:458–472.3. O'Brien MF, Stafford EG, Gardner MA, Pohlner PG, McGiffin DC. A comparison of aortic valve replacement with viable cryopreserved and fresh allograft valves, with a note on chromosomal studies. J Thorac Cardiovasc Surg. 1987. 94:812–823.4. David WC, Christopher AP, Villa K, Donna M, Lee JM, Gregory JW. Development of a pericardial acellular matrix biomaterial: Biochemical and mechanical effects of cell extraction. J Biomed Mater Res. 1994. 28:655–666.5. Bader A, Schilling T, Teebken OE, Brandes G, Herden T, Steinhoff G, Haverich A. Tissue engineering of heart valves-human endothelial cell seeding of detergent acellularized porcine valves. Eur J Cardiothorac Surg. 1998. 14:279–284.6. Teebken OE, Bader A, Steinhoff G, Haverich A. Tissue engineering of vascular grafts: human cell seeding of decellularised porcine matrix. Eur J Vasc Endovasc Surg. 2000. 19:381–386.
Article7. S Goldstein . Cryolife Inc. Treated tissue for implantation and methods of preparation. US patent. 5,632,778. 1997.8. Eybl E, Grimm M, Grabenwoger M, Bock P, Muller MM, Wolner E. Endothelial cell lining of bioprosthetic heart valve materials. J Thorac Cardiovasc Surg. 1992. 104:763–769.
Article9. Krasovskaya SM, Uzhinova LD, Andrianova MY, Prischenko AA, Livantsov MV. Biochemical and physico-chemical aspects of biomaterials calcification. Biomaterials. 1991. 12:817–820.
Article10. Gregory JW, Herman Y, Peter K, JM Lee, David WC. Acellular matrix allograft small caliber vascular prosthesis. ASAIO Trans. 1990. 36:M340–M343.11. Karl DS, Mustafa EB, Lora N, Richard T, Rajvir D, Emil AT. Homologous acellular matrix graft for urethral reconstruction in the rabbit: Histological and functional evaluation. J Urol. 2000. 163:1958–1965.12. Bodnar E, Olsen EG, Florio R, Dobrin J. Damage of porcine aortic valve tissue caused by the surfactant sodiumdodecylsulfate. Thorac Cardiovasc Surg. 1986. 34:82–85.13. Gregory JW, David WC, Peter K, Lee JM, Herman Y. Acellular matrix: A biomaterials approach for coronary artery bypass and heart valve replacement. Ann Thorac Surg. 1995. 60:S353–S358.14. Jorge-Herrero E, Fernandez P, Gutierrez M, Castillo-Olivares JL. Study of the calcification of bovine pericardium: Analysis of the implication of lipids and proteoglycans. Biomaterials. 1991. 12:683–689.
Article15. Jorge-Herrero E, Gutierrez M, Casillo-Olivares JL. Calcification of soft tissue employed in the construction of heart valve prostheses: Study of different chemical treatment. Biomaterials. 1991. 12:249–251.16. Tamura N, Nakamura T, Terai H, Iwakura A, Nomura S, Shimizu Y, Komeda M. A new acellular vascular prosthesis as a scaffold for host tissue regeneration. Int J Artif Organs. 2003. 26:783–792.17. Conklin BS, Richter ER, Kreutziger KL, Zhong DS, Chen C. Development and evaluation of a novel decellularized vascular xenograft. Med Eng Phys. 2002. 24:173–183.
Article18. Elkins RC, Goldstein S, Hewitt CW, Walsh SP, Dawson PE, Ollerenshaw JD, Black KS, Clarke DR, O'brien MF. Recellularization of heart valve grafts by a process of adaptive remodeling. Semin Thorac Cardiovasc Surg. 2001. 13(4):Supp 1. 87–92.19. Auchincloss H. Xenogeneic transplantation. A review. Transplantation. 1988. 46:1–20.20. Cozzi E, Masroor S, Soin B, Vial C, White DJ. Progress in Xenotransplantation. Clin Nephrol. 2000. 53:13–18.21. Shankarkumar U. Xenotransplantation Ethics and Immunological Hurdles! Indian J Med Sci. 2003. 57:311–318.22. Platt JL, Fishel RJ, Matas AJ, Reif SA, Bolman RM, Bach FH. Immunopathology of hyperacute xenograft rejection in a swing to primate model. Transplantation. 1991. 52:214–220.23. Dorling A, Riesbeck K, Warrens A, Lechler R. Clinical xenotransplantation of solid organs. Lancet. 1997. 349:867–871.
Article24. Morigi M, Zoja C, Colleoni S, Angioletti S, Imberti B, Remuzzi A, Remuzzi G. Xenogeneic human serum promotes leukocyte adhesion to porcine endothelium under flow conditions, possibly through the activation of the transcription factor NF-Kappa B. Xenotransplantation. 1998. 5:57–60.25. Alwayn IP, Appel JZ, Goepfert C, Bahler L, Cooper DK, Robson SC. Inhibition of platelet aggregation in baboons: therapeutic implications for xenotransplantation. Xenotransplantation. 2000. 7:247–257.
Article26. Fryer J, Firca J, Leventhal J, Blondin B, Malcom A, Ivancic D, Gandhi R, Shah A, Pao W, Abecassis M, Kaufman D, Stuart F, Anderson B. IgY antiporcine endothelial cell antibodies effectively block human antiporcine xenoantibody binding. Xenotransplantation. 1999. 6:98–109.
Article27. Joziasse DH, Oriol R. Xenotransplantation: the importance of the Gal alpha 1,3 Gal epitope in hyperacute vascular rejection. Biochim Biophys Acta. 1999. 1455:403–418.28. Bertipaglia B, Ortolani F, Petrelli L, Gerosa G, Spina M, Pauletto P, Casarotto D, Marchini M, Sartore S. Cell characterization of porcine aortic valve and decellularized leaflets repopulated with aortic valve interstitial cells: The VESALIO project (Vitalitate Exornatum Succedaneum Aorticum Labore Ingenioso Obtenibitur). Ann Thorac Surg. 2003. 75:1274–1282.
Article29. Golomb G, Schoen FJ, Smith MS, Linden J, Dixon M, Levy RJ. The role of glutaraldehyde-induced cross-links in calcification of bovine pericardium used in cardiac valve bioprosthesis. Am J Pathol. 1987. 127:122–130.30. Levy RJ, Schoen FJ, Sherman FS, Nichols J, Hawley MA. Calcification of subcutaneously implanted Type I collagen sponges: Effects of formaldehyde and glutaraldehyde. Am J Pathol. 1986. 122:71–82.31. Simon P, Kasimir MT, Seebacher G, Weigel G, Ullrich R, Salzer-Muhar U, Rieder U, Wolner E. Early failure of the tissue engineered porcine heart valve SYNERGRAFT™ in pediatric patients. Eur J Cardiothorac Surg. 2002. 21:703–710.
Article32. Cooper DK, Koren E, Oriol R. Genetically engineered pigs. Lancet. 1993. 342:682–683.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Strategies for Successful Islet Xenotransplantation
- Current Status of Solid Organ Xenotransplantation
- Histological Comparison of Vascular Grafts in a Pig to Goat Xenotransplantation Model
- Overcoming Immunological Barriers in Xenotransplantation: A Historical Review of Previous Research and Future Directions
- Regulatory macrophages as potential cell-based immunotherapy for organ xenotransplantation