J Korean Med Sci.
2007 Apr;22(2):235-241. 10.3346/jkms.2007.22.2.235.
The Increase in Hepatic Uncoupling by Fenofibrate Contributes to a Decrease in Adipose Tissue in Obese Rats
- Affiliations
-
- 1Department of Internal Medicine, Medical Science Research Institute, Dong-A University College of Medicine, 1 3-ga Dongdaesin-dong, Seo-gu, Busan, Korea. dkkim@dau.ac.kr
- 2Department of Pharmacology, Medical Science Research Institute, Dong-A University College of Medicine, Busan, Korea.
- 3Department of Pathology, Medical Science Research Institute, Dong-A University College of Medicine, Busan, Korea.
- 4Department of Radiology, Medical Science Research Institute, Dong-A University College of Medicine, Busan, Korea.
- 5Department of Anatomy, Medical Science Research Institute, Dong-A University College of Medicine, Busan, Korea.
- 6Department of Internal Medicine, Baptist Hospital, Busan, Korea.
- KMID: 1713174
- DOI: http://doi.org/10.3346/jkms.2007.22.2.235
Abstract
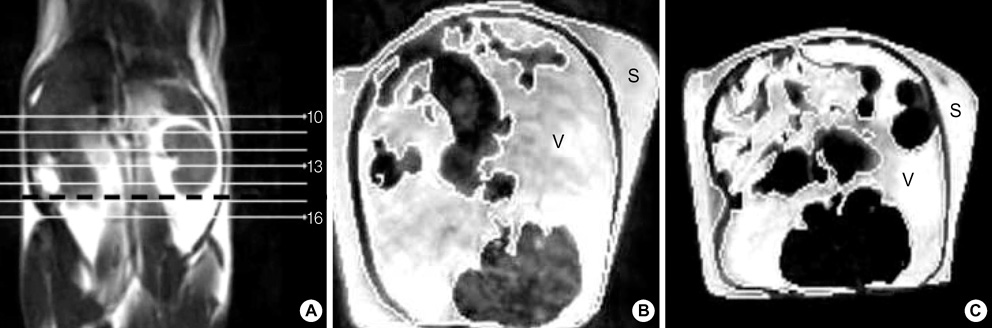
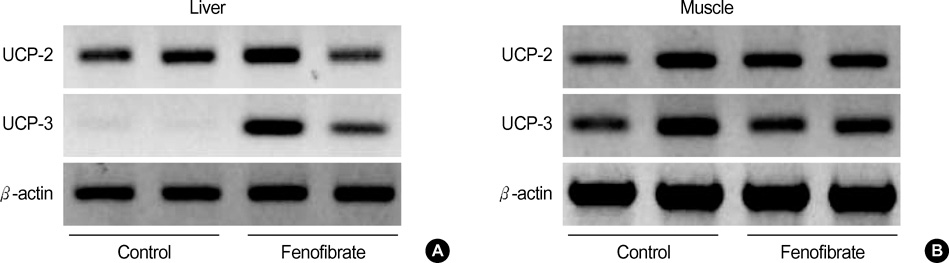
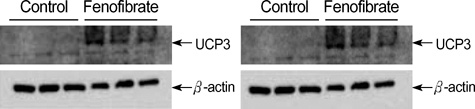
- Fenofibrate is a drug that has been suggested to inhibit weight gain by increasing the catabolism of fatty acid in the hepatic mitochondria. We hypothesized that fenofibrate induces an increase in energy expenditure in the hepatic mitochondria, which results in the reduction of adipose tissue. In this study we measured hepatic uncoupling protein (UCP)-2, -3, core temperatures and abdominal fat composition with MRI in Otsuka Long-Evans Tokushima Fatty rats. The fenofibrate group (n=7) was fed fenofibrate (320 mg/kg) mixed chow. The control group (n=7) was fed chow only. The body weight (531.6+/-7.6 g) of the fenofibrate group was significantly lower than that (744.3+/-14.9 g) of the control group (p<0.005). The areas of visceral and subcutaneous fat in the fenofibrate group (11.0+/-0.9 cm2, 4.2+/-0.3 cm2) were significantly less than those in the control group (21.0+/-0.7 cm2, 7.4+/-0.4 cm2) (p=0.046, respectively). The esophageal and rectal temperatures of the fenofibrate group (37.7+/-0.1 degrees C, 33.1+/-0.2 degrees C) were significantly higher than those of the control group (37.3+/-0.1 degrees C, 32.2+/-0.1 degrees C) (p=0.025, p=0.005). There was de novo expression of UCP-3 in the liver of the fenofibrate group. These data suggest that increased energy dissipation, via hepatic UCP-3 by fenofibrate, contribute to decreased weight gain in obese rats.
Keyword
MeSH Terms
-
Rats, Inbred OLETF
Rats
Procetofen/*pharmacology
Obesity/*physiopathology
Muscle, Skeletal/drug effects/physiopathology
Liver/drug effects/*physiopathology
Energy Metabolism/*drug effects
Body Weight/*drug effects
Body Temperature/*drug effects
Antilipemic Agents/administration & dosage
Animals
Adipose Tissue/*drug effects
Figure
Reference
-
1. Chaput E, Saladin R, Silvestre M, Edgar AD. Fenofibrate and rosiglitazone lower serum triglycerides with opposing effects on body weight. Biochem Biophys Res Commun. 2000. 271:445–450.
Article2. Mancini FP, Lanni A, Sabatino L, Moreno M, Giannino A, Contaldo F, Colantuoni V, Goglia F. Fenofibrate prevents and reduces body weight gain and adiposity in diet-induced obese rats. FEBS Lett. 2001. 491:1541–1558.
Article3. Koh EH, Kim MS, Park JY, Kim HS, Youn JY, Park HS, Youn JH, Lee KU. Peroxisome proliferator-activated receptor (PPAR)-α activation prevents diabetes in OLETF rats. Diabetes. 2003. 52:2331–2337.4. Mancini FP, Lanni A, Sabatino L, Moreno M, Giannino A, Contaldo F, Colantuoni V, Goglia F. Fenofibrate prevents and reduces body weight gain and adiposity in diet-induced obese rats. FEBS Lett. 2001. 491:154–158.
Article5. Guerre-Millo M, Gervois P, Raspe E, Madsen L, Poulain P, Derudas B, Herbert JM, Winegar DA, Willson TM, Fruchart JC, Berge RK, Staels B. Peroxisome proliferator-activated receptor α activators improve insulin sensitivity and reduce adiposity. J Biol Chem. 2000. 275:16638–16642.
Article6. Auboeuf D, Rieusset J, Fajas L, Vallier P, Frering V, Riou JP, Staels B, Auwerx J, Laville M, Vidal H. Tissue distribution and quantification of the expression of the peroxisome proliferator activated receptors and of LXR mRNAs in human: effect of obesity and NIDDM in adipose tissue. Diabetes. 1997. 46:1319–1327.7. Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998. 98:2088–2093.
Article8. Ferreira AV, Parreira GG, Green A, Botion LM. Effects of fenofibrate on lipid metabolism in adipose tissue of rats. Metabolism. 2006. 55:731–735.
Article9. Lee HJ, Choi SS, Park MK, An YJ, Seo SY, Kim MC, Hong SH, Hwang TH, Kang DY, Garber AJ, Kim DK. Fenofibrate lowers abdominal and skeletal adiposity and improves insulin sensitivity in OLETF rats. Biochem Biophys Res Commun. 2002. 296:293–299.
Article10. Larsen PJ, Jensen PB, Sorensen RV, Larsen LK, Vrang N, Wulff EM, Wassermann K. Differential influences of peroxisome proliferator-activated receptors gamma and -alpha on food intake and energy homeostasis. Diabetes. 2003. 52:2249–2259.11. Park CW, Zhang Y, Zhang X, Wu J, Chen L, Cha DR, Su D, Hwang MT, Fan X, Davis L, Striker G, Zheng F, Breyer M, Guan Y. PPARalpha agonist fenofibrate improves diabetic nephropathy in db/db mice. Kidney Int. 2006. 69:1511–1517.12. Rothwell NJ, Stock MJ. Brown adipose tissue: does it play a role in the development of obesity? Diabetes Metab Rev. 1988. 4:595–601.
Article13. Ricquier D, Bouillaud F, Toumelin P, Mory G, Bazin R, Arch J, Pénicaud L. 1986 Expression of uncoupling protein mRNA in thermogenic or weakly thermogenic brown adipose tissue. Evidence for a rapid B-adrenoreceptor-mediated and transcriptionally regulated step during activation of thermogenesis. J Biol Chem. 1986. 261:13905–13910.14. Dulloo AG, Samec S, Seydoux J. Uncoupling protein 3 and fatty acid metabolism. Biochem Soc Trans. 2001. 29:785–791.
Article15. Nisoli E, Carruba MO, Tonello C, Macor C, Federspil G, Vettor R. Induction of fatty acid translocase/CD36, peroxisome proliferator-activated receptor-gamma2, leptin, uncoupling proteins 2 and 3, and tumor necrosis factor-alpha gene expression in human subcutaneous fat by lipid infusion. Diabete. 2000. 49:319–324.
Article16. Armstrong MB, Towle HC. Polyunsaturated fatty acids stimulate hepatic UCP-2 expression via a PPAR alpha-mediated pathway. Am J Physiol Endocrinol Metab. 2001. 281:1197–1204.17. Cabrero A, Alegret M, Sanchez R, Adzet T, Laguna JC, Vazquez M. Peroxisome proliferator-activated receptor alpha (PPAR alpha) activators, bezafibrate and Wy-14,643, increase uncoupling protein-3 mRNA levels without modifying the mitochondrial membrane potential in primary culture of rat preadipocytes. Arch Biochem Biophys. 200. 380:353–359.18. Teruel T, Smith SA, Peterson J, Clapham JC. Synergistic activation of UCP-3 expression in cultured fetal rat brown adipocytes by PPAR alpha and PPAR gamma ligands. Biochem Biophys Res Commun. 2000. 273:560–564.19. Pedraza N, Solanes G, Carmona MC, Iglesias R, Vinas O, Mampel T, Vazquez M, Giralt M, Villarroya F. Impaired expression of the uncoupling protein-3 gene in skeletal muscle during lactation: fibrates and troglitazone reverse lactation-induced downregulation of the uncoupling protein-3 gene. Diabetes. 2000. 49:1224–1230.
Article20. Kelly LJ, Vicario PP, Thompson GM, Candelore MR, Doebber TW, Ventre J, Wu MS, Meurer R, Forrest MJ, Conner MW, Cascieri MA, Moller DE. Peroxisome proliferator-activated receptors gamma and alpha mediate in vivo regulation of uncoupling protein (UCP-1, UCP-2, UCP-3) gene expression. Endocrinology. 1998. 139:4920–4927.21. Berg JM, Tymoczko JL, Stryer L. The Citric Acid Cycle. Biochemistry. 2002. 5th ed. New York: WH Freeman & Company;465–490.22. Himms-Hagen J, Harper ME. Physiological Role of UCP3 May Be Export of Fatty Acids from Mitochondria When Fatty Acid Oxidation Predominates: An Hypothesis. Exp Biol Med (Maywood). 2001. 226:78–84.
Article23. Lanni A, Mancini F, Sabatino L, Silvestri E, Franco R, De Rosa G, Goglia F, Colantuoni V. De novo expression of uncoupling protein 3 is associated to enhanced mitochondrial thioesterase-1 expression and fatty acid metabolism in liver of fenofibrate-treated rats. FEBS Letters. 2002. 525:7–12.
Article24. Acin A, Rodriguez M, Rique H, Canet E, Boutin JA, Galizzi JP. Cloning and characterization of the 5' flanking region of the human uncoupling protein 3 (UCP3) gene. Biochem Biophys Res Commun. 1999. 258:278–283.25. Brun S, Carmona MC, Mampel T, Vinas O, Giralt M, Iglesias R, Villarroya F. Activators of peroxisome proliferator-activated receptor-alpha induce the expression of the uncoupling protein-3 gene in skeletal muscle: a potential mechanism for the lipid intake-dependent activation of uncoupling protein-3 gene expression at birth. Diabetes. 1999. 48:1217–1222.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fenofibrate inhibits adipocyte hypertrophy and insulin resistance by activating adipose PPARalpha in high fat diet-induced obese mice
- Prevention of Diabetes by Fenofibrate in OLETF Rats: Hepatic Mechanism for Reducing Visceral Adiposity
- Effect of Fenofibrate and Exercise on Metabolic Syndrome and Hepatic Steatosis
- Exercise and Fenofibrate Reduces Body Adiposity Synergistically in OLETF Rats
- Reduction of Food Intake by Fenofibrate is Associated with Cholecystokinin Release in Long-Evans Tokushima Rats