J Vet Sci.
2013 Dec;14(4):373-380. 10.4142/jvs.2013.14.4.373.
Time-dependent changes of calbindin D-28K and parvalbumin immunoreactivity in the hippocampus of rats with streptozotocin-induced type 1 diabetes
- Affiliations
-
- 1Department of Biomedical Laboratory Science, College of Biomedical Sciences, Soonchunhyang University, Asan 336-745, Korea. admiral96@sch.ac.kr
- KMID: 1712302
- DOI: http://doi.org/10.4142/jvs.2013.14.4.373
Abstract
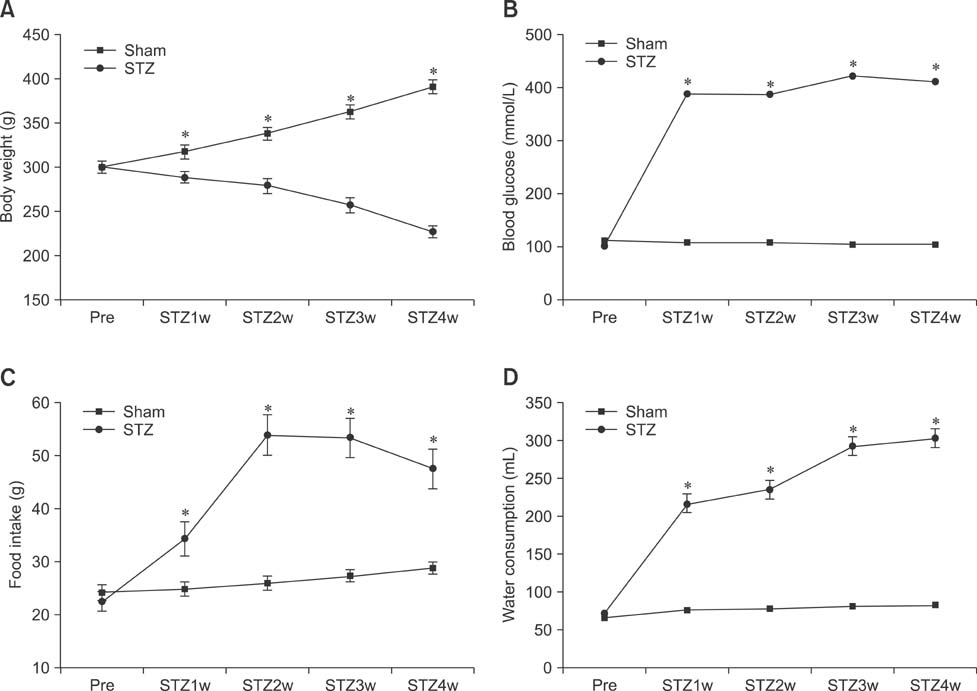
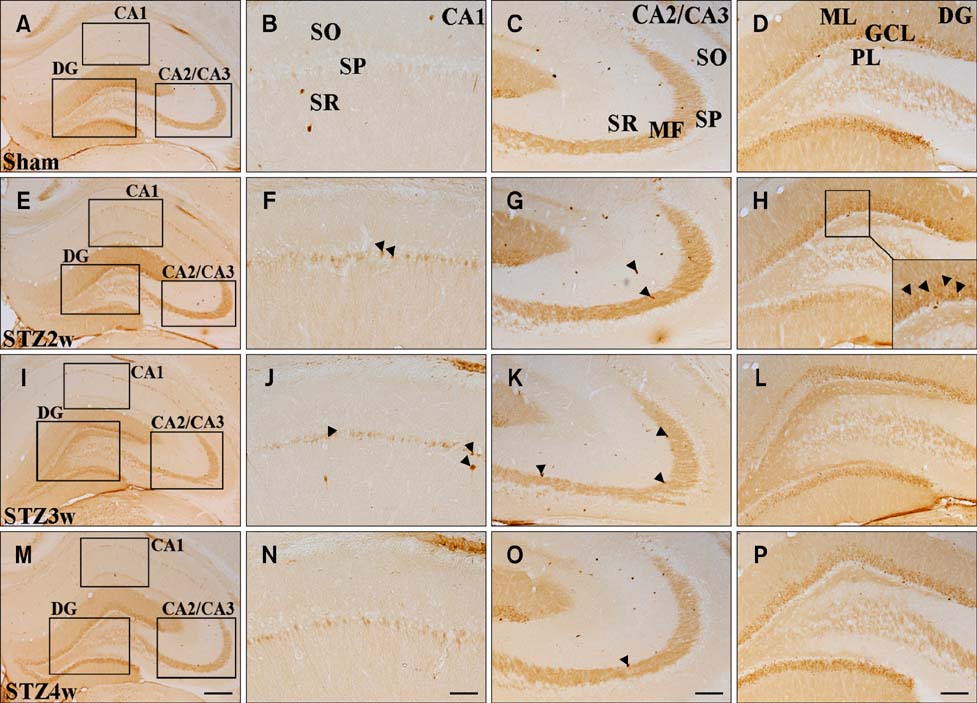
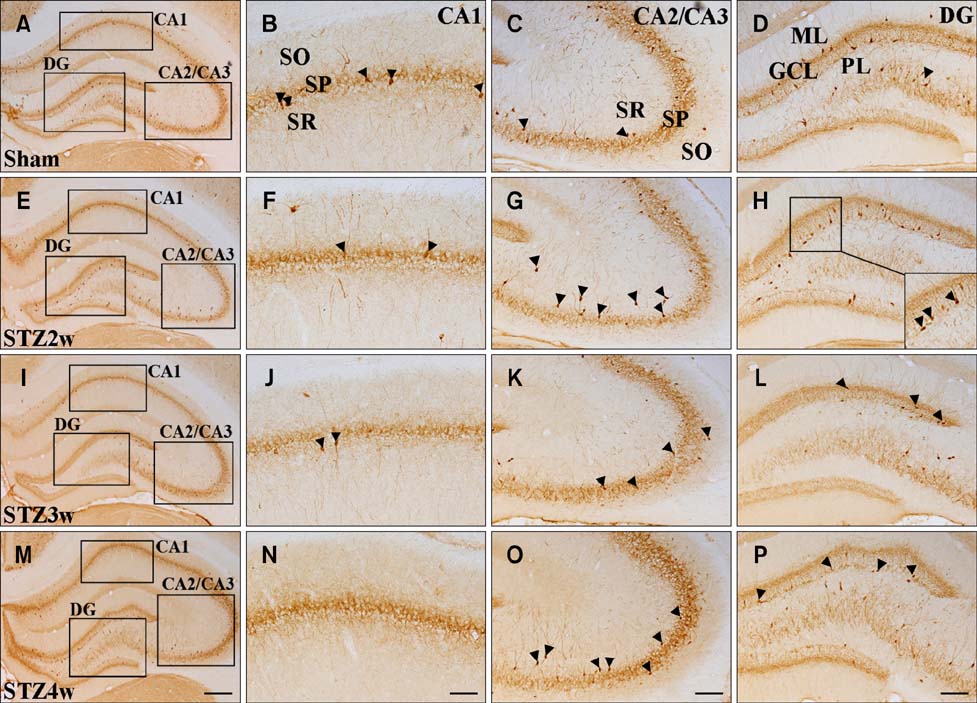
- The hippocampus is affected by various stimuli that include hyperglycemia, depression, and ischemia. Calcium-binding proteins (CaBPs) have protective roles in the response to such stimuli. However, little is known about the expression of CaBPs under diabetic conditions. This study was conducted to examine alterations in the physiological parameters with type 1 diabetes induced with streptozotocin (STZ) as well as time-dependent changes in the expression of two CaBPs changes of were being evaluated. Rats treated with STZ (70 mg/kg) had high blood glucose levels (>21.4 mmol/L) along with increased food intake and water consumption volumes compared to the sham controls. In contrast, body weight of the animals treated with STZ was significantly reduced compared to the sham group. CB-specific immunoreactivity was generally increased in the hippocampal CA1 region and granule cell layer of the dentate gyrus (DG) 2 weeks after STZ treatment, but decreased thereafter in these regions. In contrast, the number of PV-immunoreactive neurons and fibers was unchanged in the hippocampus and DG 2 weeks after STZ treatment. However, this number subsequently decreased over time. These results suggest that CB and PV expression is lowest 3 weeks after STZ administration, and these deficits lead to disturbances in calcium homeostasis.
MeSH Terms
-
Animals
Calbindin 1/*genetics/metabolism
Diabetes Mellitus, Experimental/*chemically induced
Diabetes Mellitus, Type 1/*chemically induced
*Gene Expression Regulation
Hippocampus/*metabolism
Male
Parvalbumins/*genetics/metabolism
Rats
Rats, Wistar
Streptozocin/administration & dosage
Calbindin 1
Parvalbumins
Streptozocin
Figure
Cited by 1 articles
-
Sequential alterations of glucocorticoid receptors in the hippocampus of STZ-treated type 1 diabetic rats
Jae Hoon Shin, Je Kyung Seong, Sun Shin Yi
J Vet Sci. 2014;15(1):19-26. doi: 10.4142/jvs.2014.15.1.19.
Reference
-
1. Baimbridge KG, Celio MR, Rogers JH. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992; 15:303–308.
Article2. Celio MR. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience. 1990; 35:375–475.
Article3. DeFelipe J, Hendry SHC, Jones EG. Synapses of double bouquet cells in monkey cerebral cortex visualized by calbindin immunoreactivity. Brain Res. 1989; 503:49–54.
Article4. DeFelipe J, Hendry SHC, Hashikawa T, Molinari M, Jones EG. A microcolumnar structure of monkey cerebral cortex revealed by immunocytochemical studies of double bouquet cell axons. Neuroscience. 1990; 37:655–673.
Article5. Demeulemeester H, Vandesande F, Orban GA, Brandon C, Vanderhaeghen JJ. Heterogeneity of GABAergic cells in cat visual cortex. J Neurosci. 1988; 8:988–1000.
Article6. Demeulemeester H, Vandesande F, Orban GA, Heizmann CW, Pochet R. Calbindin D-28K and parvalbumin immunoreactivity is confined to two separate neuronal subpopulations in the cat visual cortex, whereas partial coexistence is shown in the dorsal lateral geniculate nucleus. Neurosci Lett. 1989; 99:6–11.
Article7. Demeulemeester H, Arckens L, Vandesande F, Orban GA, Heizmann CW, Pochet R. Calcium binding proteins and neuropeptides as molecular markers of GABAergic interneurons in the cat visual cortex. Exp Brain Res. 1991; 84:538–544.
Article8. Freund TF, Buzsáki G, Leon A, Baimbridge KG, Somogyi P. Relationship of neuronal vulnerability and calcium binding protein immunoreactivity in ischemia. Exp Brain Res. 1990; 83:55–66.
Article9. Freund RF, Buszsáki G. Interneurons of the hippocampus. Hippocampus. 1996; 6:347–470.
Article10. Gavard JA, Lustman PJ, Clouse RE. Prevalence of depression in adults with diabetes. An epidemiological evaluation. Diabetes Care. 1993; 16:1167–1178.
Article11. Gomez R, Vargas CR, Wajner M, Barros HMT. Lower in vivo brain extracellular GABA concentration in diabetic rats during forced swimming. Brain Res. 2003; 968:281–284.
Article12. Green AR, Hainsworth AH, Jackson DM. GABA potentiation: a logical pharmacological approach for the treatment of acute ischaemic stroke. Neuropharmacology. 2000; 39:1483–1494.
Article13. Grillo CA, Piroli GG, Wood GE, Reznikov LR, McEwen BS, Reagan LP. Immunocytochemical analysis of synaptic proteins provides new insights into diabetes-mediated plasticity in the rat hippocampus. Neuroscience. 2005; 136:477–486.
Article14. Heizmann CW, Braun K. Changes in Ca2+-binding proteins in human neurodegenerative disorders. Trends Neurosci. 1992; 15:259–264.15. Hwang IK, Yoo KY, Nam YS, Choi JH, Seo K, Lee IS, Jung JY, Kang TC, Oh YS, Won MH. Age-related changes in calretinin-immunoreactive periglomerular cells in the rat main olfactory bulb. J Vet Med Sci. 2006; 68:465–469.
Article16. Iqbal Z, Ochs S. Fast axoplasmic transport of a calcium-binding protein in mammalian nerve. J Neurochem. 1978; 31:409–418.
Article17. Lee CH, Hwang IK, Yoo KY, Choi JH, Park OK, Lee JC, Jeong YG, Lee IS, Won MH. Calbindin D-28k immunoreactivity and its protein level in hippocampal subregions during normal aging in gerbils. Cell Mol Neurobiol. 2009; 29:665–672.
Article18. Leuba G, Kraftsik R, Saini K. Quantitative distribution of parvalbumin, calretinin, and calbindin D-28k immunoreactive neurons in the visual cortex of normal and Alzheimer cases. Exp Neurol. 1998; 152:278–291.
Article19. Lustman PJ, Griffith LS, Gavard JA, Clouse RE. Depression in adults with diabetes. Diabetes Care. 1992; 15:1631–1639.
Article20. Malone JI, Lowitt S, Korthals JK, Salem A, Miranda C. The effect of hyperglycemia on nerve conduction and structure is age dependent. Diabetes. 1996; 45:209–215.
Article21. Malone JI, Lowitt S, Salem AF, Miranda C, Korthals JK, Carver J. The effects of acetyl-L-carnitine and sorbinil on peripheral nerve structure, chemistry, and function in experimental diabetes. Metabolism. 1996; 45:902–907.
Article22. McEwen BS, Magariños AM, Reagan LP. Studies of hormone action in the hippocampal formation possible relevance to depression and diabetes. J Psychosom Res. 2002; 53:883–890.23. McMahon A, Wong BS, Iacopino AM, Ng MC, Chi S, German DC. Calbindin-D28k buffers intracellular calcium and promotes resistance to degeneration in PC12 cells. Brain Res Mol Brain Res. 1998; 54:56–63.
Article24. Mikkonen M, Alafuzoff I, Tapiola T, Soininen H, Miettinen R. Subfield-and layer-specific changes in parvalbumin, calretinin and calbindin-D28K immunoreactivity in the entorhinal cortex in Alzheimer's disease. Neuroscience. 1999; 92:515–532.
Article25. Molinari S, Battini R, Ferrari S, Pozzi L, Killcross AS, Robbins TW, Jouvenceau A, Billard JM, Dutar P, Lamour Y, Baker WA, Cox H, Emson PC. Deficit in memory and hippocampal long-term potentiation in mice with reduced calbindin D28K expression. Proc Natl Acad Sci U S A. 1996; 93:8028–8033.
Article26. Nam SM, Kim YN, Yoo DY, Yi SS, Kim W, Hwang IK, Seong JK, Yoon YS. Hypothyroid states mitigate the diabetes-induced reduction of calbindin D-28k, calretinin, and parvalbumin immunoreactivity in type 2 diabetic rats. Neurochem Res. 2012; 37:253–260.
Article27. Ng YK, Zeng XX, Ling EA. Expression of glutamate receptors and calcium-binding proteins in the retina of streptozotocin-induced diabetic rats. Brain Res. 2004; 1018:66–72.
Article28. Paxinos G, Watson C. The Rat Brain: In Stereotaxic Coordinates. 3rd ed. Amsterdam: Elsevier Academic Press;2007. p. 30–33.29. Perez-Pinzon MA, Yenari MA, Sun GH, Kunis DM, Steinberg GK. SNX-111, a novel, presynaptic N-type calcium channel antagonist, is neuroprotective against focal cerebral ischemia in rabbits. J Neurol Sci. 1997; 153:25–31.
Article30. Petty F. GABA and mood disorders: a brief review and hypothesis. J Affect Disord. 1995; 34:275–281.
Article31. Rothman SM, Olney JW. Excitotoxicity and the NMDA receptor-still lethal after eight years. Trends Neurosci. 1995; 18:57–58.32. Scharfman HE, Schwartzkroin PA. Protection of dentate hilar cells from prolonged stimulation by intracellular calcium chelation. Science. 1989; 246:257–260.
Article33. Shiah IS, Yatham LN. GABA function in mood disorders: an update and critical review. Life Sci. 1998; 63:1289–1303.
Article34. Sposato V, Manni L, Chaldakov GN, Aloe L. Streptozotocin-induced diabetes is associated with changes in NGF levels in pancreas and brain. Arch Ital Biol. 2007; 145:87–97.35. Steinberg GK, Panahian N, Pérez-Pinzón MA, Sun GH, Modi MW, Sepinwall J. Narrow temporal therapeutic window for NMDA antagonist protection against focal cerebral ischemia. Neurobiol Dis. 1995; 2:109–118.
Article36. Yanovsky Y, Schubring SR, Yao Q, Zhao Y, Li S, May A, Haas HL, Lin JS, Sergeeva OA. Waking action of ursodeoxycholic acid (UDCA) involves histamine and GABAA Receptor Block. PLoS One. 2012; 7:e42512.
Article37. Yenari MA, Minami M, Sun GH, Meier TJ, Kunis DM, McLaughlin JR, Ho DY, Sapolsky RM, Steinberg GK. Calbindin D28K overexpression protects striatal neurons from transient focal cerebral ischemia. Stroke. 2001; 32:1028–1035.
Article38. Yi SS, Hwang IK, Kim YN, Kim IY, Pak SI, Lee IS, Seong JK, Yoon YS. Enhanced expressions of arginine vasopressin (Avp) in the hypothalamic paraventricular and supraoptic nuclei of type 2 diabetic rats. Neurochem Res. 2008; 33:833–841.
Article39. Yi SS, Hwang IK, Chun MS, Kim YN, Kim IY, Lee IS, Seong JK, Yoon YS. Glucocorticoid receptor changes associate with age in the paraventricular nucleus of type II diabetic rat model. Neurochem Res. 2009; 34:851–858.
Article40. Yi SS, Hwang IK, Kim DW, Shin JH, Nam SM, Choi JH, Lee CH, Won MH, Seong JK, Yoon YS. The chronological characteristics of SOD1 activity and inflammatory response in the hippocampi of STZ-induced type 1 diabetic rats. Neurochem Res. 2011; 36:117–128.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Postnatal Development of Parvalbumin and Calbindin D-28k Immunoreactivities in the Canine Anterior Cingulate Cortex: Transient Expression in Layer V Pyramidal Cells
- Immunohistochemical Studies on the Calbindin D -28K and Parvalbumin Positive Neurons in the Brain Stem and Spinal Cord after Transection of Spinal Cord of Rats
- Morphological Studies on the Calbindin D-28K and Parvalbumin Immunoreactive Neurons in the Medulla Oblongata and Ventral Horn of the Spinal Cord Gray Matter after Spinal Cord Injury in Rats
- Immunocytochemical Studies of Calbindin D-28k and Parvalbumin in the Sensory and motor Cortex of the Cat
- Immunocytochemical Studies of Calbindin D-28k and Parvalbumin-Containing GABAergic Neurons in the Midbrain of the Cat