J Vet Sci.
2013 Jun;14(2):207-214. 10.4142/jvs.2013.14.2.207.
Radiation up-regulates the expression of VEGF in a canine oral melanoma cell line
- Affiliations
-
- 1Clinic for Internal Medicine and Infectious Diseases, Institute of Immunology, University of Veterinary Medicine, 1210 Vienna, Austria. Irene.flickinger@vetmeduni.ac.at
- 2Department for Pathobiology, University of Veterinary Medicine, 1210 Vienna, Austria.
- 3Small Animal Clinic Hollabrunn, 2020 Hollabrunn, Austria.
- 4Department for Natural Sciences, University of Veterinary Medicine, 1210 Vienna, Austria.
- KMID: 1705523
- DOI: http://doi.org/10.4142/jvs.2013.14.2.207
Abstract
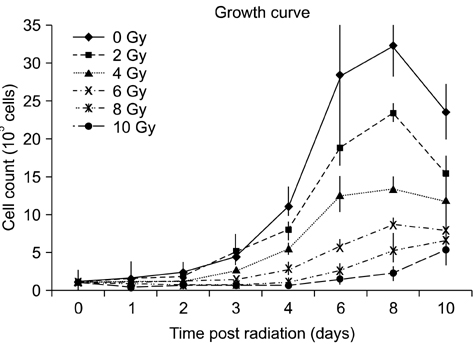
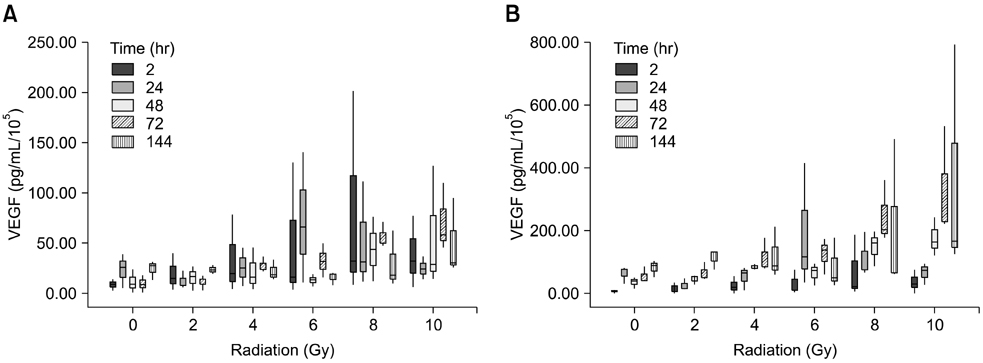
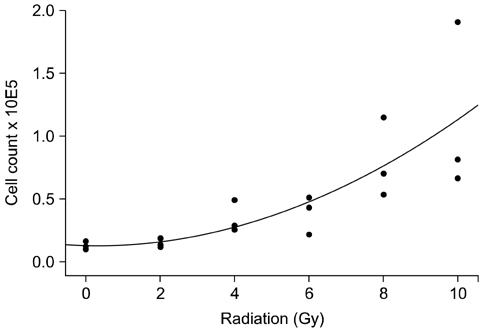
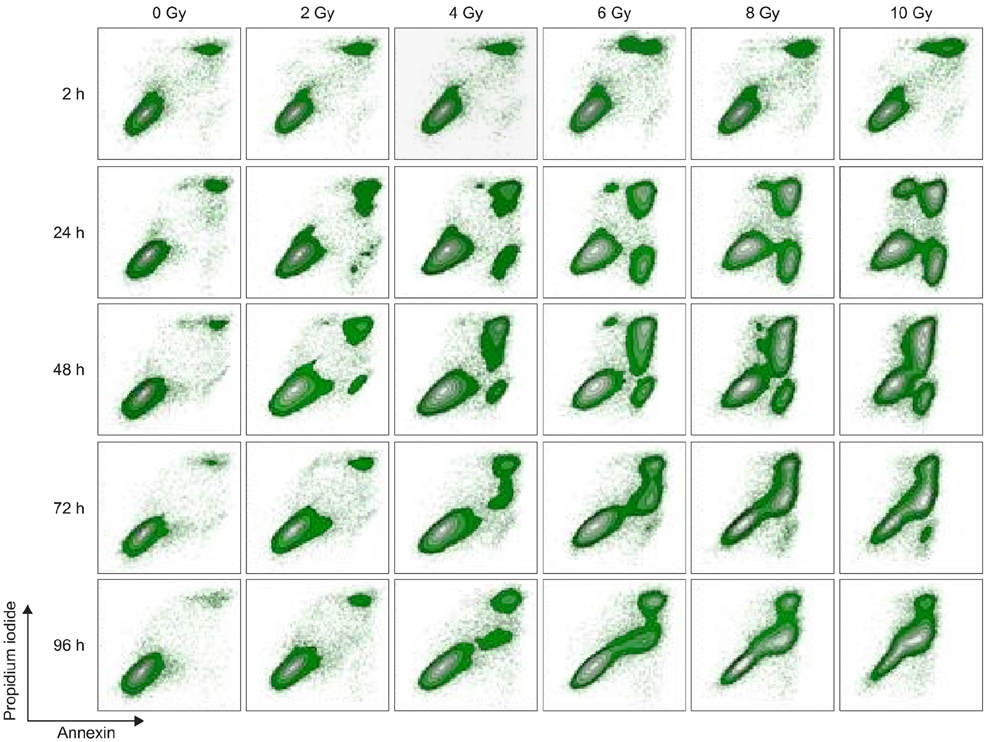
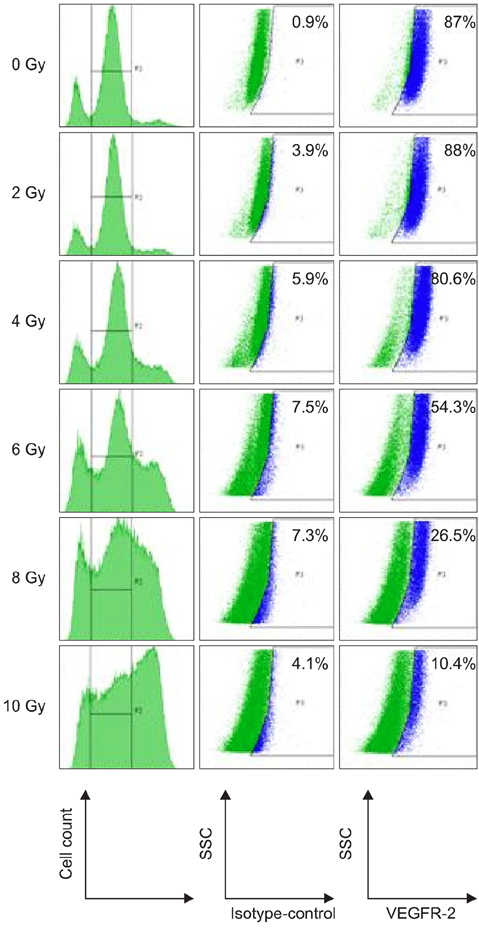
- To evaluate radiosensitivity and the effects of radiation on the expression of vascular endothelial growth factor (VEGF) and VEGF receptors in the canine oral melanoma cell line, TLM 1, cells were irradiated with doses of 0, 2, 4, 6, 8 and 10 Gray (Gy). Survival rates were then determined by a MTT assay, while vascular endothelial growth factor receptor (VEGFR)-1 and -2 expression was measured by flow cytometry and apoptotic cell death rates were investigated using an Annexin assay. Additionally, a commercially available canine VEGF ELISA kit was used to measure VEGF. Radiosensitivity was detected in TLM 1 cells, and mitotic and apoptotic cell death was found to occur in a radiation dose dependent manner. VEGF was secreted constitutively and significant up-regulation was observed in the 8 and 10 Gy irradiated cells. In addition, a minor portion of TLM 1 cells expressed vascular endothelial growth factor receptor (VEGFR)-1 intracellularly. VEGFR-2 was detected in the cytoplasm and was down-regulated following radiation with increasing dosages. In TLM 1 cells, apoptosis plays an important role in radiation induced cell death. It has also been suggested that the significantly higher VEGF production in the 8 and 10 Gy group could lead to tumour resistance.
MeSH Terms
-
Animals
Apoptosis/*radiation effects
Cell Line, Tumor/radiation effects
Dogs
Dose-Response Relationship, Radiation
Enzyme-Linked Immunosorbent Assay/veterinary
Melanoma/genetics/metabolism
Mouth Neoplasms/genetics/metabolism
Radiation Tolerance
Tetrazolium Salts/metabolism
Thiazoles/metabolism
Up-Regulation/*radiation effects
Vascular Endothelial Growth Factor A/genetics/metabolism/*radiation effects
Vascular Endothelial Growth Factor Receptor-1/genetics/metabolism/*radiation effects
Vascular Endothelial Growth Factor Receptor-2/genetics/metabolism/*radiation effects
Tetrazolium Salts
Thiazoles
Vascular Endothelial Growth Factor A
Vascular Endothelial Growth Factor Receptor-1
Vascular Endothelial Growth Factor Receptor-2
Figure
Reference
-
1. Bateman KE, Catton PA, Penncock PW, Kruth SA. 0-7-21 radiation therapy for treatment of canine oral melanoma. J Vet Intern Med. 1994; 8:267–272.2. Bergman PJ, McKnight J, Novosad A, Charney S, Farrelly J, Craft D, Wulderk M, Jeffers Y, Sadelain M, Hohenhaus AE, Segal N, Gregor P, Engelhorn M, Riviere I, Houghton AN, Wolchok JD. Long-term survival of dogs with advanced malignant melanoma after DNA vaccination with xenogeneic human tyrosinase: a phase I trial. Clin Cancer Res. 2003; 9:1284–1290.3. Berkman RA, Merrill MJ, Reinhold WC, Monacci WT, Saxena A, Clark WC, Robertson JT, Ali IU, Oldfield EH. Expression of the vascular permeability factor/vascular endothelial growth factor gene in central nervous system neoplasms. J Clin Invest. 1993; 91:153–159.
Article4. Blackwood L, Dobson JM. Radiotherapy of oral malignant melanomas in dogs. J Am Vet Med Assoc. 1996; 209:98–102.5. Brodey RS. Canine and feline neoplasia. Adv Vet Sci Comp Med. 1970; 14:309–354.6. Chaudhry IH, O'Donovan DG, Brenchley PEC, Reid H, Roberts ISD. Vascular endothelial growth factor expression correlates with tumor grade and vascularity in gliomas. Histopathology. 2001; 39:409–415.
Article7. Clifford CA, Hughes D, Beal MW, Mackin AJ, Henry CJ, Shofer FS, Sorenmo KU. Plasma vascular endothelial growth factor concentrations in healthy dogs and dogs with hemangiosarcoma. J Vet Intern Med. 2001; 15:131–135.
Article8. Endlich B, Radford IR, Forrester HB, Dewey WC. Computerized video time-lapse microscopy studies of ionizing radiation-induced-rapid-interphase and mitosis-related apoptosis in lymphoid cells. Radiat Res. 2000; 153:36–48.
Article9. Ferrara N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr Rev. 2004; 25:581–611.
Article10. Ferrara N, Gerber HP, Le Couter J. The biology of VEGF and its receptors. Nat Med. 2003; 9:669–676.
Article11. Ferrara N, Vis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997; 18:4–25.
Article12. Gampel A, Moss L, Jones MC, Brunton V, Norman JC, Mellor H. VEGF regulates the mobilization of VEGFR2/KDR from an intracellular endothelial storage compartement. Blood. 2006; 108:2624–2631.
Article13. Goldschmidt MH. Benign and malignant melanocytic neoplasms of domestic animals. Am J Dermatopathol. 1985; 7:203–212.
Article14. Gorski DH, Beckett MA, Jaskowiak NT, Calvin DP, Mauceri HJ, Salloum RM, Seetharam S, Koons A, Hari DM, Kufe DW, Weichselbaum RR. Blockade of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999; 59:3374–3378.15. Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005; 23:1011–1027.
Article16. Kowanetz M, Ferrara N. Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res. 2006; 12:5018–5022.
Article17. Marino DJ, Matthiesen DT, Stefanacci JD, Mororff SD. Evaluation of dogs with digit masses: 117 cases (1981-1991). J Am Vet Med Assoc. 1995; 207:726–728.18. McEntee MC. Summary of Results of Cancer Treatment with radiation therapy. In : Morrison WB, editor. Cancer in Dogs and Cats: Medical and Surgical Management. 2nd ed. Jackson: Teton NewMedia;2002. p. 389–424.19. Modiano JF, Ritt MG, Wojcieszyn J. The molecular basis of canine melanoma: pathogenesis and trends in diagnosis and therapy. J Vet Intern Med. 1999; 13:163–174.
Article20. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.
Article21. Moultan JE. Tumors in Domestic Animals. 3rd ed. Berkeley: University of California Press;1990. p. 75–87.22. Park JS, Qiao L, Su ZZ, Hinman D, Willoughby K, McKinstry R, Yacoub A, Duigou GJ, Young CSH, Grant S, Hagan MP, Ellis E, Fisher PB, Dent P. Ionizing radiation modulates vascular endothelial growth factor (VEGF) expression through multiple mitogen activated protein kinase dependent pathways. Oncogene. 2001; 20:3266–3280.
Article23. Platt SR, Scase TJ, Adams V, Wieczorek L, Miller J, Adamo F, Long S. Vascular endothelial growth factor expression in canine intracranial meningiomas and association with patient survival. J Vet Intern Med. 2006; 20:663–668.
Article24. Rebuzzi L, Willmann M, Sonneck K, Gleixner KV, Florian S, Kondo R, Mayerhofer M, Vales A, Gruze A, Pickl WF, Thalhammer JG, Valent P. Detection of vascular endothelial growth factor (VEGF) and VEGF receptors Flt-1 and KDR in canine mastocytoma cells. Vet Immunol Immunopathol. 2007; 115:320–333.
Article25. Sekis I, Gerner W, Willmann M, Rebuzzi L, Tichy A, Patzl M, Thalhammer JG, Saalmüller A, Kleiter MM. Effect of radiation on vascular endothelial growth factor expression in the C2 canine mastocytoma cell line. Am J Vet Res. 2009; 70:1141–1150.
Article26. Taylor KH, Smith AN, Higginbotham M, Schwartz DD, Carpenter DM, Whitley EM. Expression of vascular endothelial growth factor in canine oral malignant melanoma. Vet Comp Oncol. 2007; 5:208–218.
Article27. Todoroff RJ, Brodey RS. Oral and pharyngeal neoplasia in the dog: a retrospective survey of 361 cases. J Am Vet Med Assoc. 1979; 175:567–571.28. Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol. 2001; 19:577–583.
Article29. Wergin MC, Ballmer-Hofer K, Roos M, Achermann RE, Inteeworn N, Akens MK, Blattmann H, Kaser-Hotz B. Preliminary study of plasma vascular endothelial growth factor (VEGF) during low- and high-dose radiation therapy of dogs with spontaneous tumors. Vet Radiol Ultrasound. 2004; 45:247–254.
Article30. Withrow SJ, MacEwen EG. Withrow's and MacEwen's Small Animal Clinical Oncology. 3rd ed. St. Louis: W. B. Saunders;2001. p. 305–317.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stage-specific embryonic antigen: determining expression in canine glioblastoma, melanoma, and mammary cancer cells
- VEGF Expression and Microvessel Density in Oral Squamous Cell Carcinomas
- The Prognostic Effect of VEGF Expression in Squamous Cell Carcinoma of the Cervix Treated with Radiation Therapy Alone
- Preferential Cytotoxic Effect of Genistein on G361 Melanoma Cells Via Inhibition of the Expression of Focal Adhesion Kinase
- Expression of vascular endothelial growth factor in oral squamous cell carcinoma