J Korean Soc Spine Surg.
2007 Mar;14(1):1-7. 10.4184/jkss.2007.14.1.1.
Notochordal Cells Induce Chemotaxis of Cartilage-Endplate Chondrocytes in In Vitro Motility Assays
- Affiliations
-
- 1Department of Orthopedic Surgery, College of Medicine, The Catholic University of Korea, Seoul, Korea. kiwonk@lycos.co.kr
- 2Department of Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Department of Orthopedic Surgery, Dongshin General Hospital, Seoul, Korea.
- KMID: 1527137
- DOI: http://doi.org/10.4184/jkss.2007.14.1.1
Abstract
-
STUDY DESIGN: In vitro motility assays were carried out using rat intervertebral discs (IVDs).
OBJECTIVES
To demonstrate the motile properties of the cartilage endplate (CE) chondrocytes and the effect of notochordal cells on this property. LITERATURE REVIEW: Although previous in vivo studies have provided evidence for the migration of CE chondrocyte from hyaline CEs into the notochordal nucleus pulposus (NP), it is unclear if CE chondrocytes of the IVD actually have motile properties. In addition, the effect of notochordal cells on these properties has not been reported.
MATERIALS AND METHODS
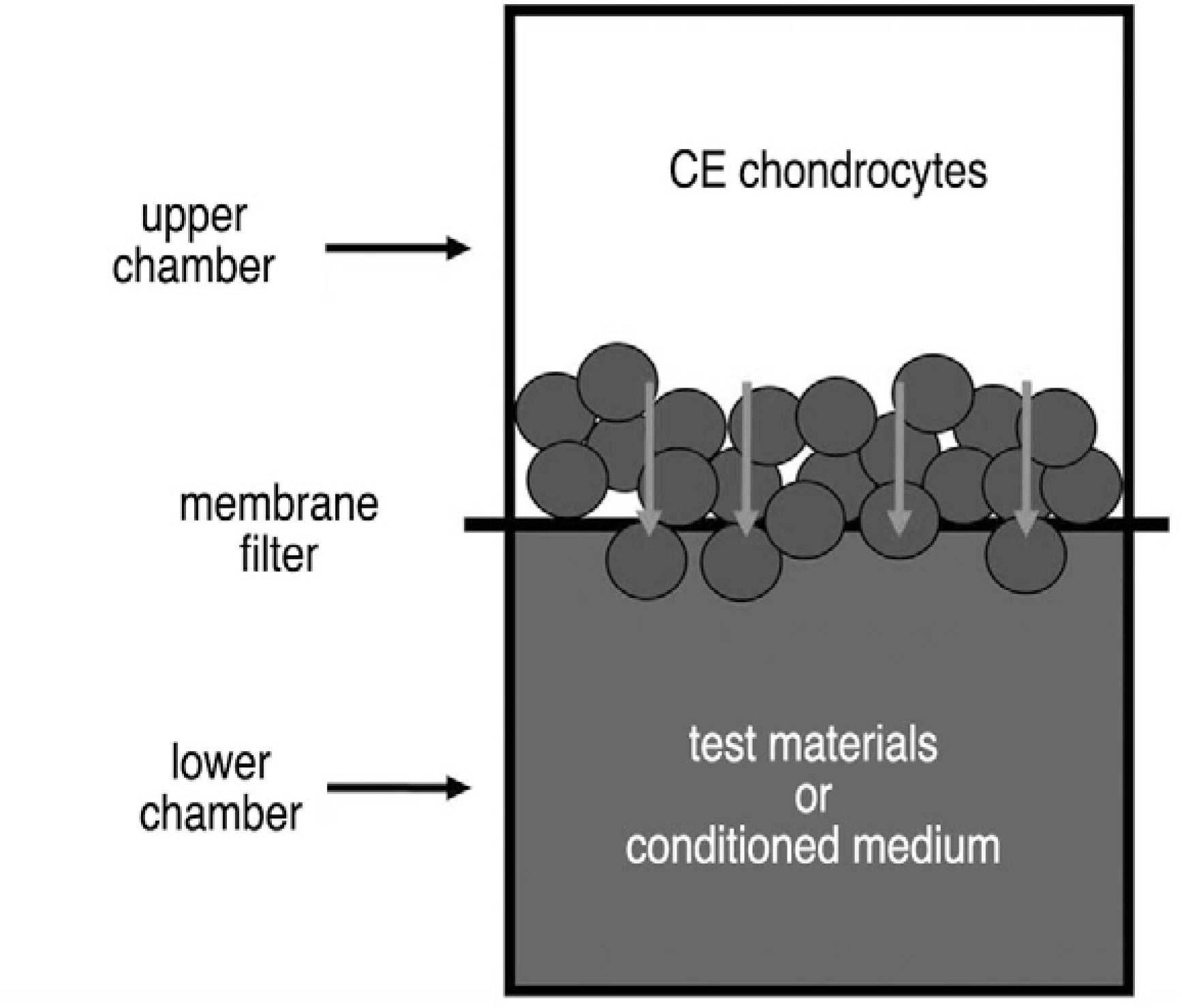
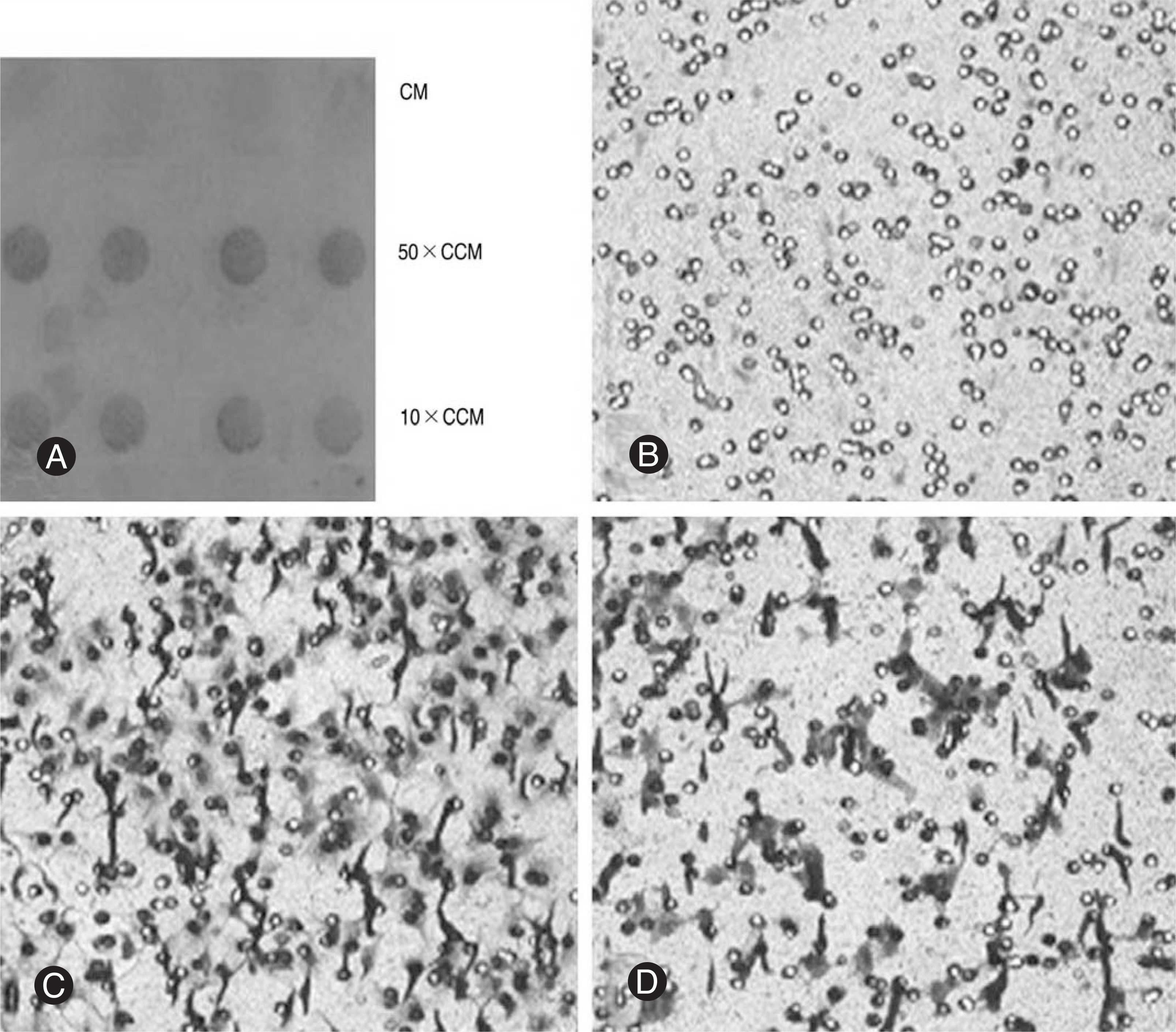
Notochordal cells and CE chondrocytes were harvested from three-month-old male Wistar rats and cultured separately. The motility was assayed in quadruplicate using a 48-well microchemotaxis chamber and a gelatin-coated 8-micrometer polycarbonate membrane filter. The control medium (serum-free culture medium), notochordal cells (4x, 2x, 1x and 0.5x10(6)) and concentrated conditioned medium (10-, 50-fold) where notochordal cells were cultured were loaded into the wells of the lower chamber, and CE chondrocytes were added to the wells of the upper chamber. At the end of the assays, the CE chondrocytes that migrated to the bottom side of the membrane filter were stained, counted, and compared.
RESULTS
Compared with the control medium, the notochordal cells (N = 4x, 2x, 1x and 0.5x10(6)) and concentrated conditioned medium (10- and 50-fold) significantly increased the chemotactic motility of the CE chondrocytes in a number- and concentration-dependent manner (p<0.05).
CONCLUSION
The CE chondrocytes of the intervertebral disc are motile, and soluble factors produced by notochordal cells induce the chemotaxis of CE chondrocytes.
MeSH Terms
Figure
Reference
-
1). Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995; 20:1307–1314.
Article2). Kim KW, Lim TH, Kim JG, Jeong ST, Masuda K, An HS. The origin of chondrocytes in the nucleus pulposus and histologic findings associated with the transition of a notochordal nucleus pulposus to a fibrocartilaginous nucleus pulposus in intact rabbit intervertebral discs. Spine. 2003; 28:982–990.
Article3). Peacock A. Observations on the postnatal structure of the intervertebral disc in man. J Anat. 1952; 86:162–179.4). Kim KW, Ha KY, Park JB, Woo YK, Chung HN, An HS. Expressions of Membrane-Type I Matrix Metallopro-teinase, Ki-67 Protein, and Type II Collagen by Chondrocytes Migrating from Cartilage Endplate into Nucleus Pulposus in Rat Intervertebral Discs: A Cartilage End-plate-Fracture Model Using an Intervertebral Disc Organ Culture. Spine. 2005; 30:1373–1378.5). Singer SJ, Kupfer A. The directed migration of eukaryot-ic cells. Annu Rev Cell Biol. 1986; 2:337–365.
Article6). Aznavoorian S, Stracke ML, Krutzsch H, Schiffmann E, Liotta LA. Signal transduction for chemotaxis and haptotaxis by matrix molecules in tumor cells. J Cell Biol. 1990; 110:1427–1438.
Article7). Alini M, Li W, Markovic P, Aebi M, Spiro RC, Rough-ley PJ. The potential and limitations of a cell-seeded collagen/hyaluronan scaffold to engineer an intervertebral disc-like matrix. Spine. 2003; 28:446–454.
Article8). Gan JC, Ducheyne P, Vresilovic EJ, Shapiro IM. Intervertebral disc tissue engineering II: cultures of nucleus pulposus cells. Clin Orthop. 2003; 411:315–324.
Article9). Masuda K, Takegami K, An H, et al. Recombinant osteogenic protein-1 upregulates extracellular matrix metabolism by rabbit annulus fibrosus and nucleus pulposus cells cultured in alginate beads. J Orthop Res. 2003; 21:922–930.
Article10). Nam SW, Clair T, Campo CK, Lee HY, Liotta LA, Stracke ML. Autotaxin (ATX), a potent tumor motogen, augments invasive and metastatic potential of ras-trans-formed cells. Oncogene. 2000; 19:241–247.
Article11). Nam SW, Clair T, Schiffmann E, Liotta LA, Stracke ML. A sensitive screening assay for secreted motility-stimulating factors. Cell Motil Cytoskeleton. 2000; 46:279–284.
Article12). Hama K, Aoki J, Fukaya M, et al. Lysophosphatidic acid and autotaxin stimulate cell motility of neoplastic and non-neoplastic cells through LPA1. J Biol Chem. 2004; 279:17634–17639.
Article13). Kim KW, Kim YS, Ha KY, et al. An autocrine or paracrine Fas-mediated counterattack: a potential mechanism for apoptosis of notochordal cells in intact rat nucleus pulposus. Spine. 2005; 30:1247–1251.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Repair of Osteochondral Defect Using Grafts of Cultured Chondrocytes in Rabbits
- Mesenchymal Stem Cell-Specific Chemokines for Articular Cartilage Repair
- Disc-Type Hyaline Cartilage Reconstruction Using 3D-Cell Sheet Culture of Human Bone Marrow Stromal Cells and Human Costal Chondrocytes and Maintenance of Its Shape and Phenotype after Transplantation
- In Vitro Tissue Engineering of Cartilage using Autologous Fibrin Glue and Chondrocytes
- Effects of Tissue Transglutaminase on Human Chondrocytes Adhesion to Degenerated Articular Cartilage