J Korean Rheum Assoc.
2007 Jun;14(2):144-148. 10.4078/jkra.2007.14.2.144.
Severe Leukopenia after Intravenous Cyclophosphamide Pulse Therapyin a Patient Having Cytochrome P450 2A6*1B
- Affiliations
-
- 1Department of Internal Medicine, The Hospital for Rheumatic Diseases, Hanyang University College of Medicine, Seoul, Korea. scbae@hanyang.ac.kr
- 2Department of Pharmacology and Pharmacogenomics Research Center, Inje University College of Medicine, Busan, Korea.
- 3Department of Internal Medicine, Konyang University, Daejeon, Korea.
- KMID: 1526431
- DOI: http://doi.org/10.4078/jkra.2007.14.2.144
Abstract
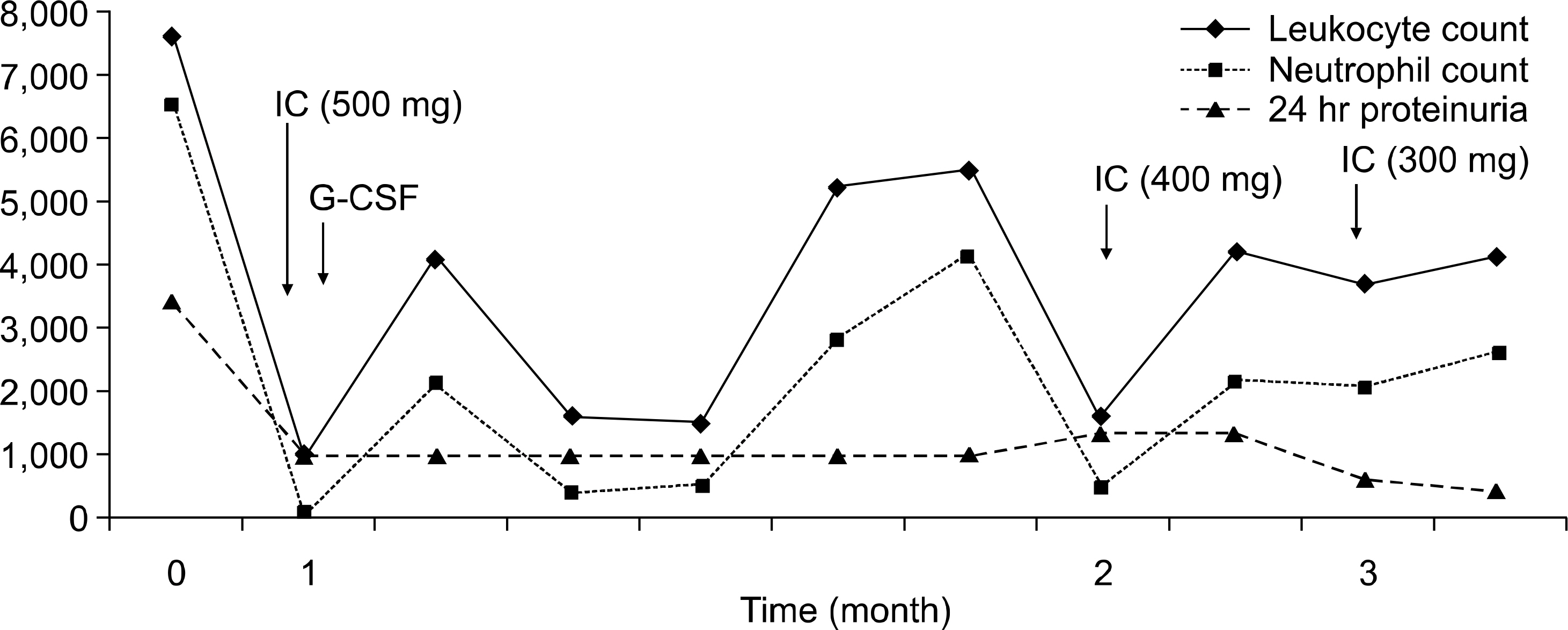
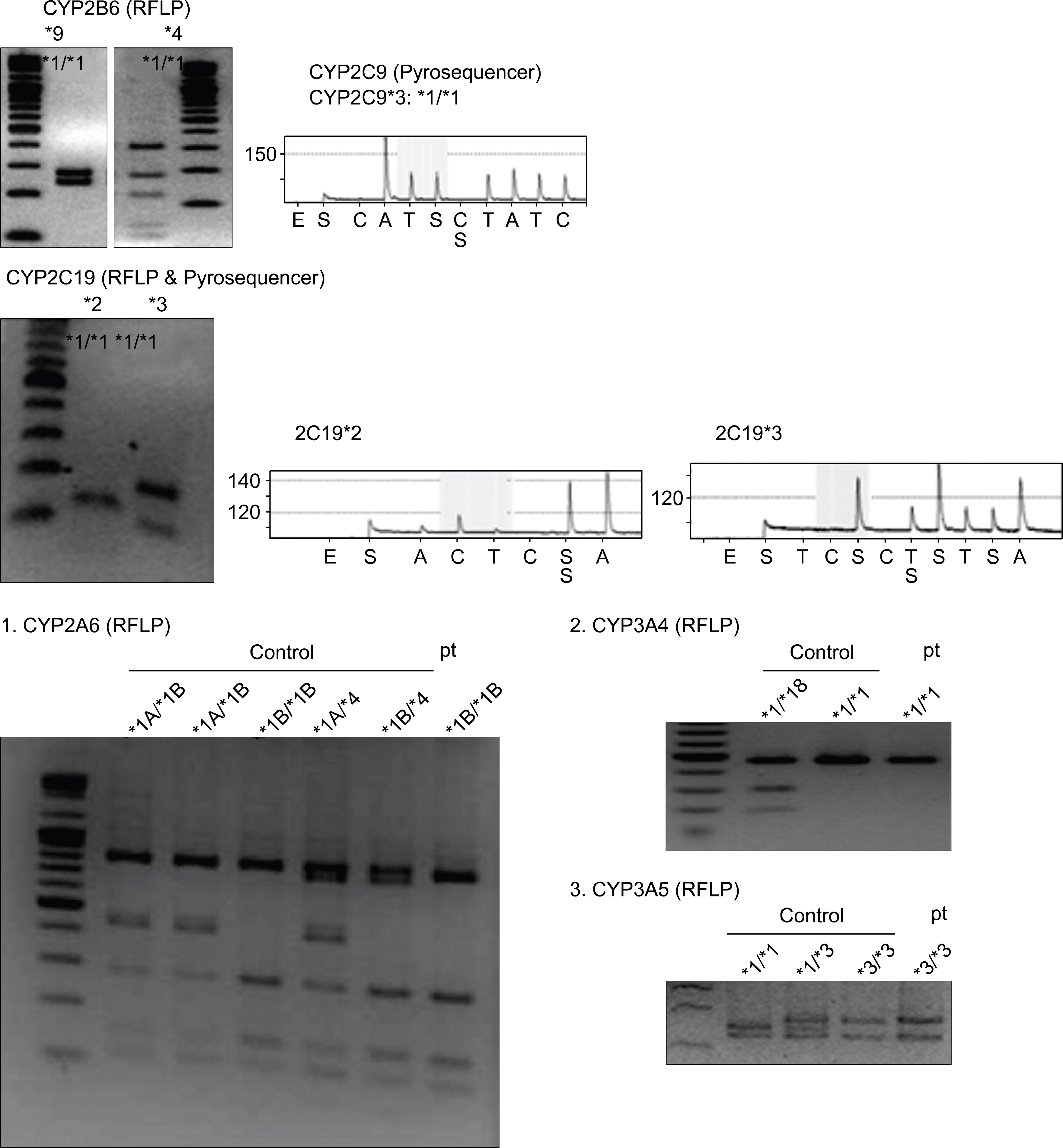
- Cyclophosphamide, a prodrug requiring metabolic activation by cytochrome P450 (CYP) enzymes, is used widely for proliferative lupus nephritis and various CYP isoenzymes have been demonstrated to be involved in the bioactivation of cyclophosphamide in humans, including CYP2A6, 2B6, 2C19, 2C9, 3A4, and 3A5. The response or adverse event after intravenous cyclophosphamide pulse therapy in lupus nephritis patient seems to be different for each individual and genetic polymorphism of CYP may explain the difference. Generally, wild types of CYP seem to be more active in the activation of cyclophosphamide than variant types of CYP. Here, we report a case of lupus nephritis with a genotype of CYP2A6*1B who suffered from severe leukopenia after intravenous cyclophosphamide pulse therapy.
Keyword
MeSH Terms
Figure
Reference
-
1). Houssiau FA. Cyclophosphamide in lupus nephritis. Lupus. 2005. 14:53–8.
Article2). Katsifis GE., Tzioufas AG., Vlachoyiannopoulos PG., Voulgarelis M., Moutsopoulos HM., Ioannidis JP. Risk of myelotoxicity with intravenous cyclopho-sphamide in patients with systemic lupus erythematosus. Rheumatology. 2002. 41:780–6.
Article3). Takada K., Arefayene M., Desta Z., Yarboro CH., Boumpas DT., Balow JE, et al. Cytochrome P450 pharmacogenetics as a predictor of toxicity and clinical response to pulse cyclophosphamide in lupus nephritis. Arthritis Rheum. 2004. 50:2202–10.
Article4). Colvin M., Padgett CA., Fenselau C. A biologically active metabolite of cyclophosphamide. Cancer Res. 1973. 33:915–8.5). Fenselau C., Kan MN., Rao SS., Myles A., Friedman ᄋM., Colvin M. Identification of aldophos-phamide as a metabolite of cyclophosphamide in vitro and in vivo in humans. Cancer Res. 1977. 37:2538–43.6). Friedman ᄋM., Wodinsky I., Myles A. Cyclophosphamide (NSC-26271)-related phosphoramide mustards-recent advances and historical perspective. Cancer Treat Rep. 1976. 60:337–46.7). Connors TA., Cox PJ., Farmer PB., Foster AB., Jarman M. Some studies of the active intermediates formed in the microsomal metabolism of cyclophosphamide and isophosphamide. Biochem Pharmacol. 1974. 23:115–29.
Article8). de Jonge ME., Huitema AD., Rodenhuis S., Beijnen JH. Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet. 2005. 44:1135–64.
Article9). Forrester LM., Henderson CJ., Glancey MJ., Back DJ., Park BK., Ball SE, et al. Relative expression of cytochrome P450 isoenzymes in human liver and association with the metabolism of drugs and xenobiotics. Biochem J. 1992. 281:359–68.
Article10). Nasu R., Matsuo H., Takanaga H., ᄋhtani H., Sawada Y. Quantitative prediction of catalepsy induced by amoxapine, cinnarizine and cyclophosphamide in mice. Biopharm Drug Dispos. 2000. 21:129–38.
Article11). Shimada T., Yamazaki H., Mimura M., Inui Y., Guengerich FP. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994. 270:414–23.12). Suh JW., Koo BK., Zhang SY., Park KW., Cho JY., Jang IJ, et al. Increased risk of atherothrombotic events associated with cytochrome P450 3A5 polymorphism in patients taking clopidogrel. Cmaj. 2006. 174:1715–22.
Article13). Hustert E., Haberl M., Burk ᄋ., Wolbold R., He YQ., Klein K, et al. The genetic determinants of the CYP3A5 polymorphism. Pharmacogenetics. 2001. 11:773–9.
Article14). Lee SJ., Usmani KA., Chanas B., Ghanayem B., Xi T., Hodgson E, et al. Genetic findings and functional studies of human CYP3A5 single nucleotide polymorphisms in different ethnic groups. Pharmacogenetics. 2003. 13:461–72.
Article15). Nakajima M., Kwon JT., Tanaka N., Zenta T., Yamamoto Y., Yamamoto H, et al. Relationship between interindividual differences in nicotine metabolism and CYP2A6 genetic polymorphism in humans. Clin Pharmacol Ther. 2001. 69:72–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Water Intoxication Following Low-Dose Intravenous Cyclophosphamide
- Inhibition of Cytochrome P450 Enzymes by Drugs—Molecular Basis and Practical Applications
- Cytochrome P450 and Cancer
- Inhibitory Effects of 6-Gingerol on Cytochrome P450 in Human Liver Microsomes
- Effect of intravenous pulse cyclophosphamide in the treatment of Churg-Strauss syndrome with refractory neuropathy to high-dose steroid treatment