Cancer Res Treat.
2007 Sep;39(3):93-98.
The Efficacy of an Induction Chemotherapy Combination with Docetaxel, Cisplatin, and 5-FU Followed by Concurrent Chemoradiotherapy in Advanced Head and Neck Cancer
- Affiliations
-
- 1Department of Hemato-Oncology, Chonnam National University Medical School, Gwangju, Korea. sh115@chollian.net
- 2Department of Otorhinolaryngology-Head and Neck Surgery, Chonnam National University Medical School, Gwangju, Korea.
- 3Department of Radiation Oncology, Chonnam National University Medical School, Gwangju, Korea.
Abstract
-
PURPOSE: This study was performed to determine the feasibility and safety of the use of induction chemotherapy combined with docetaxel, cisplatin, and 5-fluorouracil (TPF) followed by concurrent chemoradiation therapy for locally advanced squamous cell carcinoma of the head and neck (SCCHN).
MATERIALS AND METHODS
The patients, that were initially not treated for locally advanced SCCHN, underwent three cycles of induction chemotherapy every 3 weeks at a dose of 70 mg/m2 docetaxel D1, 75 mg/m2 cisplatin D1, 1000 mg/m2 5-FU D1-4, and subsequently received concurrent chemoradiation therapy.
RESULTS
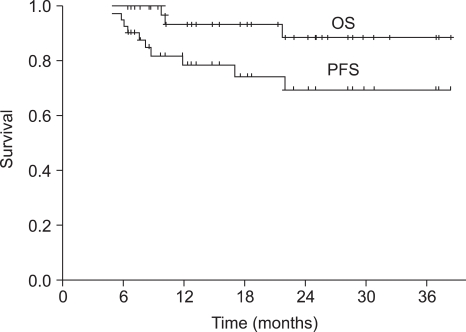
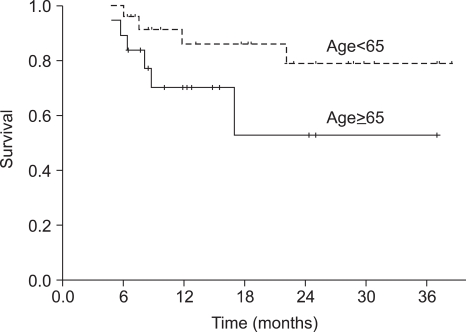
Forty-nine patients were enrolled in this study and forty-three of the patients completed the treatment. The median duration of follow-up was 18 months (range, 6~39 months). All of the patients had stage III (26.5%) or IV (73.5%) squamous cell carcinoma. After sequential therapy, a complete response and partial response was seen in 28 (65.2%) and 13 (30.2%) patients, respectively. The overall response rate was 95.4%. Overall survival and progression-free survival (PFS) at 2 years were 88.7% and 69.7%, respectively. Grade 3~4 neutropenia occurred in 42.2% of the patients and grade 4 thrombocytopenia in 1 cycle (0.7%). Two patients (4.1%) died during the induction chemotherapy due to pneumonia and a subdural hemorrhage, respectively. The group of patients over 65 years of age showed a significant lower dose intensity than that of patients under 65 years of age, but PFS was not significantly different between two groups (p=0.105).
CONCLUSION
TPF induction chemotherapy followed by concurrent chemoradiotherapy showed a high level of CR and moderate treatment-induced toxicity. Adequate dose modification in elderly patients should be considered to maintain efficacy and avoid treatment-related toxicity.
MeSH Terms
Figure
Reference
-
1. Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001; 345:1890–1900. PMID: 11756581.
Article2. Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006; 56:106–130. PMID: 16514137.
Article3. Lefebvre JL, Chevalier D, Luboinski B, Kirkpatrick A, Collette L, Sahmoud T. Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst. 1996; 88:890–899. PMID: 8656441.4. Guadagnolo BA, Haddad RI, Posner MR, Weeks L, Wirth LJ, Norris CM, et al. Organ preservation and treatment toxicity with induction chemotherapy followed by radiation therapy or chemoradiation for advanced laryngeal cancer. Am J Clin Oncol. 2005; 28:371–378. PMID: 16062079.
Article5. Pignon JP, Bourhis J, Domenge C, Designe L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000; 355:949–955. PMID: 10768432.6. Janinis J, Papadakou M, Xidakis E, Boukis H, Poulis A, Panagos G, et al. Combination chemotherapy with docetaxel, cisplatin, and 5-fluorouracil in previously treated patients with advanced/recurrent head and neck cancer: a phase II feasibility study. Am J Clin Oncol. 2000; 23:128–131. PMID: 10776971.7. Catimel G, Verweij J, Mattijssen V, Hanauske A, Piccart M, Wanders J, et al. EORTC Early Clinical Trials Group. Docetaxel (Taxotere): an active drug for the treatment of patients with advanced squamous cell carcinoma of the head and neck. Ann Oncol. 1994; 5:533–537. PMID: 7918125.
Article8. Lee JH, Lee KW, Choi YJ, Choi JH, Shin HJ, Chung JS, et al. Docetaxel and cisplatin combination chemotherapy in patients with squamous cell carcinomas of the head and neck. Cancer Res Treat. 2003; 35:261–266.
Article9. Hitt R, Paz-Ares L, Brandariz A, Castellano D, Pena C, Millan JM, et al. Induction chemotherapy with paclitaxel, cisplatin and 5-fluorouracil for squamous cell carcinoma of the head and neck: long-term results of a phase II trial. Ann Oncol. 2002; 13:1665–1673. PMID: 12377658.10. Hitt R, Lopez-Pousa A, Martinez-Trufero J, Escrig V, Carles J, Rizo A, et al. Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol. 2005; 23:8636–8645. PMID: 16275937.
Article11. Diaz JF, Andreu JM. Assembly of purified GDP-tubulin into microtubules induced by taxol and taxotere: reversibility, ligand stoichiometry, and competition. Biochemistry. 1993; 32:2747–2755. PMID: 8096151.
Article12. Haldar S, Basu A, Croce CM. Bcl2 is the guardian of microtubule integrity. Cancer Res. 1997; 57:229–233. PMID: 9000560.13. Posner MR, Glisson B, Frenette G, Al-Sarraf M, Colevas AD, Norris CM, et al. Multicenter phase I-II trial of docetaxel, cisplatin, and fluorouracil induction chemotherapy for patients with locally advanced squamous cell cancer of the head and neck. J Clin Oncol. 2001; 19:1096–1104. PMID: 11181674.14. Haddad R, Colevas AD, Tishler R, Busse P, Goguen L, Sullivan C, et al. Docetaxel, cisplatin, and 5-fluorouracil-based induction chemotherapy in patients with locally advanced squamous cell carcinoma of the head and neck: the Dana Farber Cancer Institute experience. Cancer. 2003; 97:412–418. PMID: 12518365.15. Colevas AD, Busse PM, Norris CM, Fried M, Tishler RB, Poulin M, et al. Induction chemotherapy with docetaxel, cisplatin, fluorouracil, and leucovorin for squamous cell carcinoma of the head and neck: a phase I/II trial. J Clin Oncol. 1998; 16:1331–1339. PMID: 9552034.
Article16. Colevas AD, Norris CM, Tishler RB, Fried MP, Gomolin HI, Amrein P, et al. Phase II trial of docetaxel, cisplatin, fluorouracil, and leucovorin as induction for squamous cell carcinoma of the head and neck. J Clin Oncol. 1999; 17:3503–3511. PMID: 10550148.
Article17. Colevas AD, Norris CM, Tishler RB, Lamb CC, Fried MP, Goguen LA, et al. Phase I/II trial of outpatient docetaxel, cisplatin, 5-fluorouracil, leucovorin (opTPFL) as induction for squamous cell carcinoma of the head and neck (SCCHN). Am J Clin Oncol. 2002; 25:153–159. PMID: 11943893.
Article18. Posner MR, Haddad RI, Wirth L, Norris CM, Goguen LA, Mahadevan A, et al. Induction chemotherapy in locally advanced squamous cell cancer of the head and neck: evolution of the sequential treatment approach. Semin Oncol. 2004; 31:778–785. PMID: 15599855.
Article19. Tae K, Keum HS, Kang SY, Lee HS, Choi JH, Kim IS, et al. Neoadjuvant chemotherapy followed by concurrent chemoradiation in locally advanced head and neck squamous cell carcinoma. Korean J Otolaryngol-Head Neck Surg. 2007; 50:327–334.20. Vokes EE. Kasper DL, Braunwald E, Fauci AS, Hauser ST, Longo DL, Jameson JL, editors. Head and neck cancer. Harrison's principles of internal medicine. 2004. 16th ed. Seoul: McGraw-Hill;p. 501.21. Gomez H, Mas L, Casanova L, Pen DL, Santillana S, Valdivia S, et al. Elderly patients with aggressive non-Hodgkin's lymphoma treated with CHOP chemotherapy plus granulocytemacrophage colony-stimulating factor: identification of two age subgroups with differing hematologic toxicity. J Clin Oncol. 1998; 16:2352–2358. PMID: 9667250.22. Lyman GH, Dale DC, Crawford J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J Clin Oncol. 2003; 21:4524–4531. PMID: 14673039.
Article23. Dees EC, O'Reilly S, Goodman SN, Sartorius S, Levine MA, Jones RJ, et al. A prospective pharmacologic evaluation of age-related toxicity of adjuvant chemotherapy in women with breast cancer. Cancer Invest. 2000; 18:521–529. PMID: 10923100.
Article24. Schild SE, Stella PJ, Geyer SM, Bonner JA, McGinnis WL, Mailliard JA, et al. The outcome of combined-modality therapy for stage III non-small-cell lung cancer in the elderly. J Clin Oncol. 2003; 21:3201–3206. PMID: 12874270.
Article25. Choi YJ, Chung JS, Shin HJ, Cho GJ, Wang SG, Lee BJ, et al. Induction chemotherapy of docetaxel and cisplatin for the elderly patients with squamous cell carcinoma of the head and neck. Cancer Res Treat. 2007; 39:1–5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Organ Preservation for the Management of Locally Advanced Head and Neck Cancer
- Establishment of Cisplatin Resistant Head and Neck Cancer Cell Lines and Cross-resistance of Docetaxel
- Induction chemotherapy in patients with locally-advanced head and neck squamous cell carcinoma: docetaxel and cisplatin
- Induction Chemotherapy of Docetaxel and Cisplatin for the Elderly Patients with Squamous Cell Carcinoma of the Head and Neck
- Docetaxel and Cisplatin Combination Chemotherapy in Patients with Advanced Head and Neck Cancer