J Bacteriol Virol.
2008 Dec;38(4):167-172. 10.4167/jbv.2008.38.4.167.
Production and Quality Control of Adenoviral Vectors for Clinical Trials
- Affiliations
-
- 1Department of Microbiology, College of Medicine, the Catholic University of Korea, Seoul, Korea. yssohn@abxign.com, paik@catholic.ac.kr
- KMID: 1483956
- DOI: http://doi.org/10.4167/jbv.2008.38.4.167
Abstract
- The importance of recombinant adenoviral vectors for the development of gene therapy and prophylactic and therapeutic vaccines has led to efforts for process development of large scale production of clinically safe adenoviral vectors. First of all, cell lines producing replication incompetent adenoviral vectors required for clinical application have been developed and the concept of banking and characterization of cell lines and adenoviral vectors has been established. In order to meet the need of amount of adenoviral vectors for clinical trials, various large scale suspension culture methods using serum-free media have been developed along with development of large scale purification methods using chromatography instead of cesium chloride method. In addition, methods for the quality control of adenoviral vectors have been established and applied for the clinical lots.
MeSH Terms
Figure
Reference
-
1). Barouch DH., Nabel GL. Adenovirus vector-based vaccines for human immunodeficiency virus type 1. Hum Gene Ther. 16:149–156. 2005.
Article2). Blanche F., Cameron B., Barbot A., Ferrero L., Guillemin T., Guyot S., Somarriba S., Bisch D. An improved anion-exchange HPLC method for the detection and purification of adenoviral particles. Gene Ther. 7:1055–1062. 2000.
Article3). Boyer JL., Kobinger G., Wilson JM., Crystal RG. Adeno-virus based genetic vaccines for biodefence. Hum Gene Ther. 16:157–168. 2005.4). Croyle MA., Anderson DJ., Roessler BJ., Amidon GL. Development of a highly efficient purification process for recombinant adenoviral vectors for oral gene delivery. Pharm Dev Technol. 3:365–372. 1998.
Article5). Danthinne X., Imperiale MJ. Production of first generation adenovirus vectors: a review. Gene Ther. 7:1707–1714. 2000.
Article6). Fallaux FJ., Bout A., van der Velde I., van den Wolenberg DJ., Hehir KM., Keegan J., Auger C., Cramer SJ., van Ormondt H., van der Eb AJ., Valerio D., Hoeben RC. New helper cells and matched early region 1-deleted adenovirus vectors prevent generation of replication-competent adeno-viruses. Hum Gene Ther. 9:1909–1917. 1998.
Article7). Graham FL., Smiley J., Russell WC., Nairn R. Characterization of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 36:59–74. 1977.8). Huyghe BG., Liu X., Sutjipto S., Sugarman BJ., Horn MT., Shepard HM., Scandella CJ., Shabram P. Purification of a type 5 recombinant adenovirus encoding human p53 by column chromatography. Hum Gene Ther. 6:1403–1416. 1995.
Article9). Journal of Gene Medicine web site. www.wiley.co.uk/genemed/clinical.10). Kamen A., Henry O. Development and optimization of an adenovirus production process. J Gene Med. 6:S184–192. 2004.
Article11). Lusky M. Good manufacturing practice production of adenoviral vectors for clinical trials. Hum Gene Ther. 16:281–291. 2005.
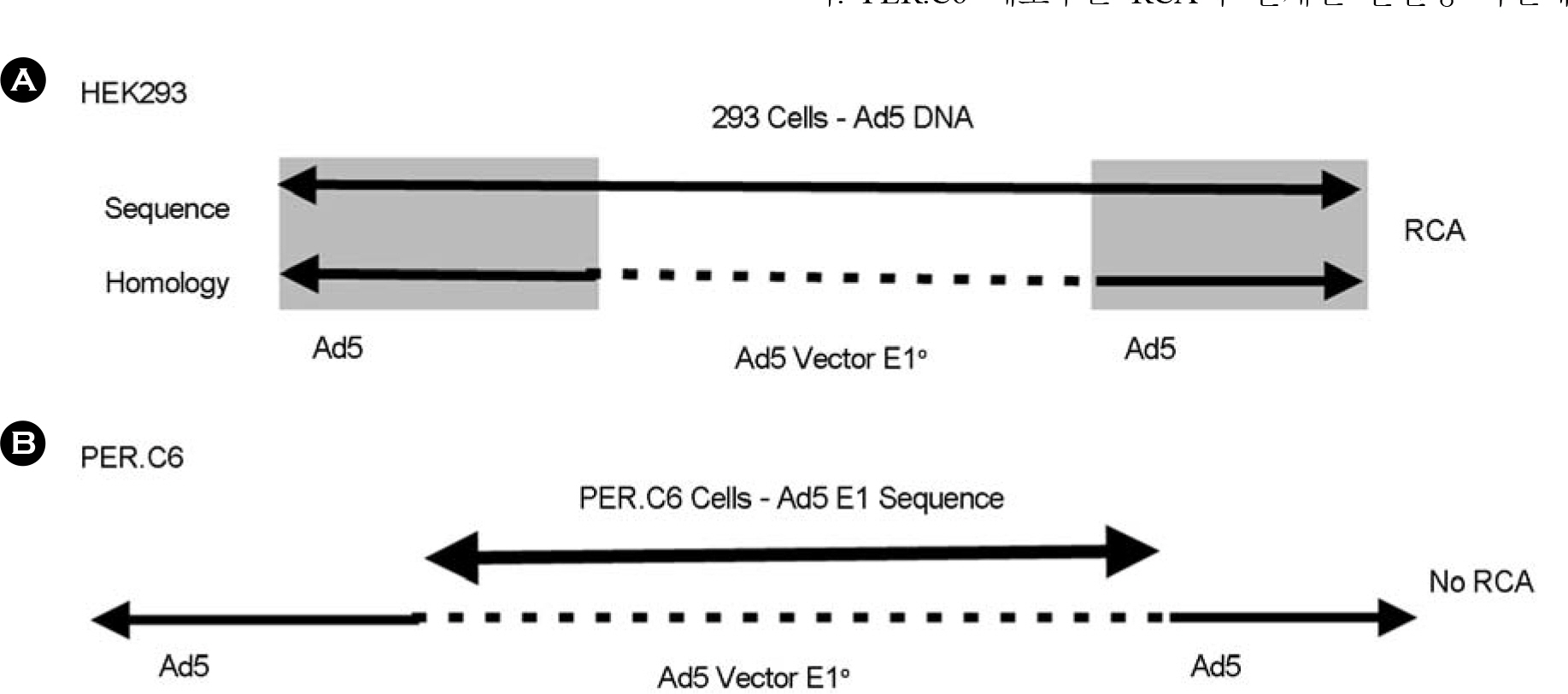
Article12). Murakami P., Pungor E., Files J., Do L., Van Rijnsoever R., Vogels R., Bout A., McCaman M. A single short stretch of homology between adenoviral vector and packaging cell line can give rise to cytopathic effect-inducing, helper-dependent E1-positive particles. Hum Gene Ther. 13:909–920. 2002.
Article13). Peixoto C., Ferreira TB., Carrondo MJ., Cruz PE., Alves PM. Purification of adenoviral vectors using expanded bed chromatography. J Virol Methods. 132:121–126. 2006.
Article14). Wilson J. Gendicine: the first commercial gene therapy product. Hum Gene Ther. 16:1014–1015. 2005.15). Wisher M. Biosafety and product release testing issues relevant to replication-competent oncolytic viruses. Cancer Gene Ther. 9:1056–1061. 2002.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecular Approaches for Brain Tumor Therapy;Gene Transfer and Anti-sense Oligonucleotides
- Development of a packaging cell line for propagation of replication-deficient adenovirus vector
- Distribution of Adenoviral Vector in Brain after Intravenous Administration
- Attenuation of the Expression of Fibrogenic Molecules by Transforming Growth Factor-beta1 Antisense in Cultured Rat Mesangial Cells
- Enhanced bone morphogenic protein adenoviral gene delivery to bone marrow stromal cells using magnetic nanoparticle