J Korean Assoc Oral Maxillofac Surg.
2013 Jun;39(3):112-119. 10.5125/jkaoms.2013.39.3.112.
Enhanced bone morphogenic protein adenoviral gene delivery to bone marrow stromal cells using magnetic nanoparticle
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, School of Dentistry, Kyungpook National University, Daegu, Korea. kwondk@knu.ac.kr
- 2Department of Biochemistry, Kyungpook National University School of Medicine, Daegu, Korea.
- KMID: 1430478
- DOI: http://doi.org/10.5125/jkaoms.2013.39.3.112
Abstract
OBJECTIVES
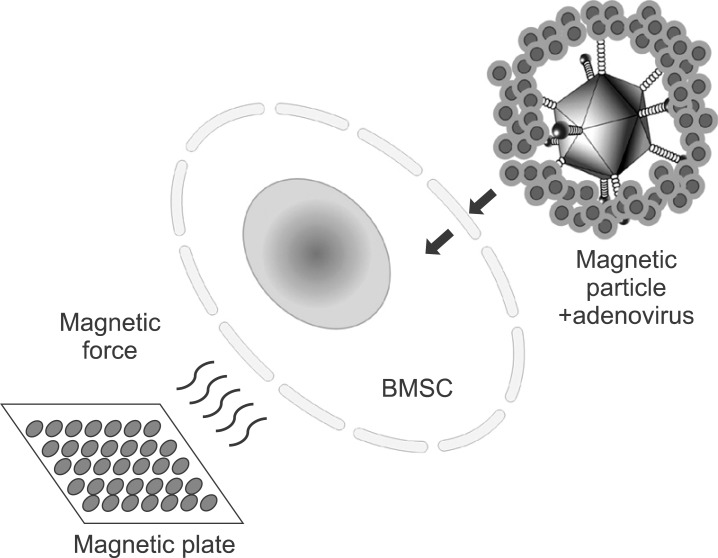
This study investigated the question of whether adenoviral magnetofection can be a suitable method for increasing the efficacy of gene delivery into bone marrow stromal cell (BMSC) and for generation of a high level of bone morphogenic protein (BMP) secretion at a minimized viral titer.
MATERIALS AND METHODS
Primary BMSCs were isolated from C57BL6 mice and transduced with adenoviral vectors encoding beta galactosidase or BMP2 and BMP7. The level of BMP secretion, activity of osteoblast differentiation, and cell viability of magnetofection were measured and compared with those of the control group.
RESULTS
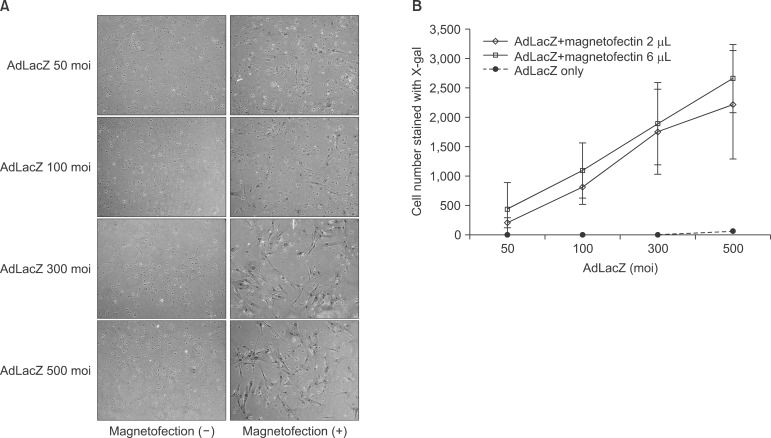
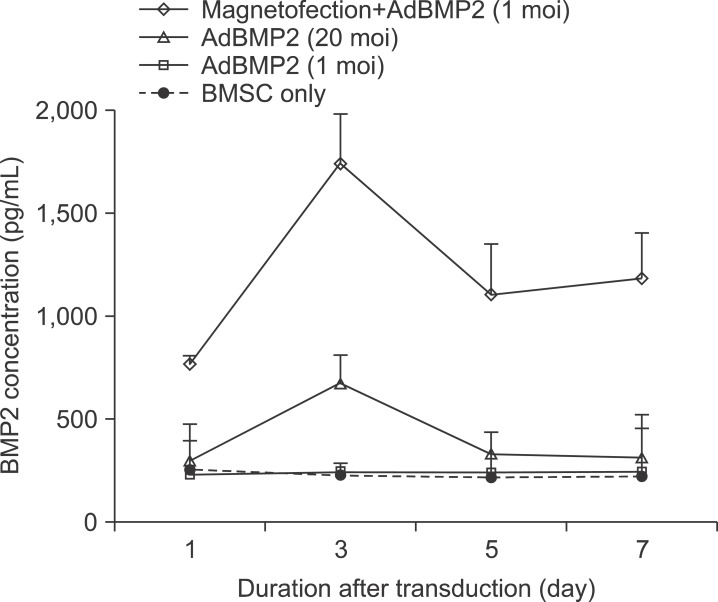
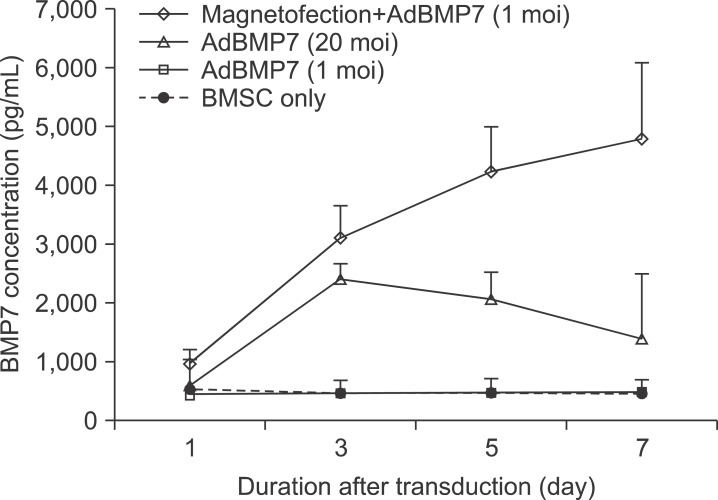
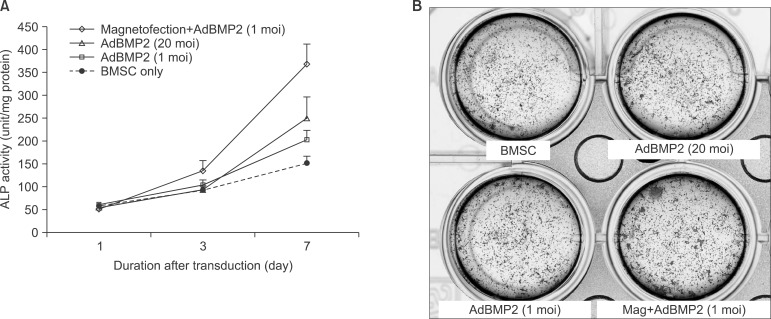
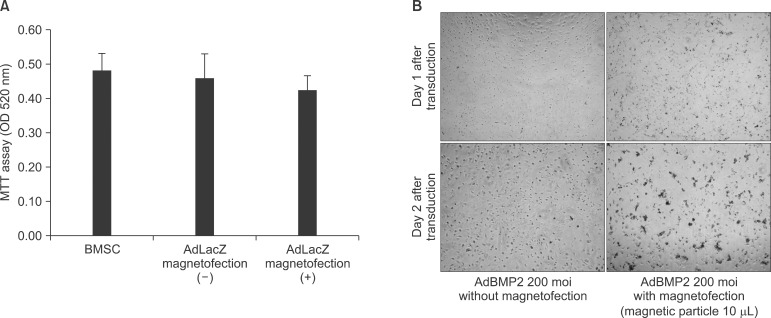
The expression level of beta galactosidase showed that the cell transduction efficiency of AdLacZ increased according to the increased amount of magnetic nanoparticles. No change in cell viability was observed after magnetofection with 2 microL of magnetic nanoparticle. Secretion of BMP2 or BMP7 was accelerated after transduction of AdBMP2 and 7 with magnetofection. AdBMP2 adenoviral magnetofection resulted in up to 7.2-fold higher secretion of BMP2, compared with conventional AdBMP2-transduced BMSCs. Magnetofection also induced a dramatic increase in secretion of BMP7 by up to 10-fold compared to the control. Use of only 1 multiplicity of infection (moi) of magnetofection with adenoviral transduction of AdBMP2 or AdBMP7 resulted in significantly higher transgene expression compared to 20 moi of conventional adenoviral transduction.
CONCLUSION
Magnetic particle-mediated gene transudation is a highly efficient method of gene delivery to BMSCs. Magnetofection can lower the amount of viral particles while improving the efficacy of gene delivery.
MeSH Terms
Figure
Reference
-
1. Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001; 19:180–192. PMID: 11359943.
Article2. Scherer F, Anton M, Schillinger U, Henke J, Bergemann C, Krüger A, et al. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002; 9:102–109. PMID: 11857068.
Article3. Plank C, Scherer F, Schillinger U, Bergemann C, Anton M. Magnetofection: enhancing and targeting gene delivery with superparamagnetic nanoparticles and magnetic fields. J Liposome Res. 2003; 13:29–32. PMID: 12725725.
Article4. Edgell CJ, Curiel DT, Hu PC, Marr HS. Efficient gene transfer to human endothelial cells using DNA complexed to adenovirus particles. Biotechniques. 1998; 25:264–268. 270–272. PMID: 9714887.
Article5. Tanner FC, Carr DP, Nabel GJ, Nabel EG. Transfection of human endothelial cells. Cardiovasc Res. 1997; 35:522–528. PMID: 9415297.
Article6. Krötz F, de Wit C, Sohn HY, Zahler S, Gloe T, Pohl U, et al. Magnetofection--a highly efficient tool for antisense oligonucleotide delivery in vitro and in vivo. Mol Ther. 2003; 7:700–710. PMID: 12718913.
Article7. Gazit D, Turgeman G, Kelley P, Wang E, Jalenak M, Zilberman Y, et al. Engineered pluripotent mesenchymal cells integrate and differentiate in regenerating bone: a novel cell-mediated gene therapy. J Gene Med. 1999; 1:121–133. PMID: 10738576.
Article8. Franceschi RT, Wang D, Krebsbach PH, Rutherford RB. Gene therapy for bone formation: in vitro and in vivo osteogenic activity of an adenovirus expressing BMP7. J Cell Biochem. 2000; 78:476–486. PMID: 10861845.
Article9. Gottschalk S, Sparrow JT, Hauer J, Mims MP, Leland FE, Woo SL, et al. A novel DNA-peptide complex for efficient gene transfer and expression in mammalian cells. Gene Ther. 1996; 3:448–457. PMID: 9156807.10. Haines AM, Irvine AS, Mountain A, Charlesworth J, Farrow NA, Husain RD, et al. CL22 - a novel cationic peptide for efficient transfection of mammalian cells. Gene Ther. 2001; 8:99–110. PMID: 11313779.
Article11. Mehrara BJ, Saadeh PB, Steinbrech DS, Dudziak M, Spector JA, Greenwald JA, et al. Adenovirus-mediated gene therapy of osteoblasts in vitro and in vivo. J Bone Miner Res. 1999; 14:1290–1301. PMID: 10457261.
Article12. Olmsted EA, Blum JS, Rill D, Yotnda P, Gugala Z, Lindsey RW, et al. Adenovirus-mediated BMP2 expression in human bone marrow stromal cells. J Cell Biochem. 2001; 82:11–21. PMID: 11400159.
Article13. Lieberman JR, Daluiski A, Stevenson S, Wu L, McAllister P, Lee YP, et al. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am. 1999; 81:905–917. PMID: 10428121.
Article14. Breitbart AS, Grande DA, Mason JM, Barcia M, James T, Grant RT. Gene-enhanced tissue engineering: applications for bone healing using cultured periosteal cells transduced retrovirally with the BMP-7 gene. Ann Plast Surg. 1999; 42:488–495. PMID: 10340856.15. Verma IM, Somia N. Gene therapy-promises, problems and prospects. Nature. 1997; 389:239–242. PMID: 9305836.16. Partridge K, Yang X, Clarke NM, Okubo Y, Bessho K, Sebald W, et al. Adenoviral BMP-2 gene transfer in mesenchymal stem cells: in vitro and in vivo bone formation on biodegradable polymer scaffolds. Biochem Biophys Res Commun. 2002; 292:144–152. PMID: 11890685.
Article17. Franceschi RT, Yang S, Rutherford RB, Krebsbach PH, Zhao M, Wang D. Gene therapy approaches for bone regeneration. Cells Tissues Organs. 2004; 176:95–108. PMID: 14745239.
Article18. Russell WC. Update on adenovirus and its vectors. J Gen Virol. 2000; 81:2573–2604. PMID: 11038369.
Article19. Zhao M, Zhao Z, Koh JT, Jin T, Franceschi RT. Combinatorial gene therapy for bone regeneration: cooperative interactions between adenovirus vectors expressing bone morphogenetic proteins 2, 4, and 7. J Cell Biochem. 2005; 95:1–16. PMID: 15759283.
Article20. Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003; 80:148–158. PMID: 14567964.
Article21. Reid T, Warren R, Kirn D. Intravascular adenoviral agents in cancer patients: lessons from clinical trials. Cancer Gene Ther. 2002; 9:979–986. PMID: 12522437.
Article22. Marshall E. Gene therapy death prompts review of adenovirus vector. Science. 1999; 286:2244–2245. PMID: 10636774.
Article23. Bergelson JM. Receptors mediating adenovirus attachment and internalization. Biochem Pharmacol. 1999; 57:975–979. PMID: 10796067.
Article24. Hung SC, Lu CY, Shyue SK, Liu HC, Ho LL. Lineage diffe-rentiation-associated loss of adenoviral susceptibility and Coxsackie-adenovirus receptor expression in human mesenchymal stem cells. Stem Cells. 2004; 22:1321–1329. PMID: 15579649.
Article25. Sapet C, Laurent N, de Chevigny A, Le Gourrierec L, Bertosio E, Zelphati O, et al. High transfection efficiency of neural stem cells with magnetofection. Biotechniques. 2011; 50:187–189. PMID: 21486240.
Article26. Sapet C, Pellegrino C, Laurent N, Sicard F, Zelphati O. Magnetic nanoparticles enhance adenovirus transduction in vitro and in vivo. Pharm Res. 2012; 29:1203–1218. PMID: 22146803.
Article27. Veiseh O, Gunn JW, Zhang M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv Drug Deliv Rev. 2010; 62:284–304. PMID: 19909778.
Article28. Plank C, Vlaskou D, Schillinger U, Mykhaylyk O. MagnetofectionTM platform: from magnetic nanoparticles to novel nucleic acid therapeutics. Ther Deliv. 2011; 2:717–726. PMID: 22822504.29. Zhang Y, Li W, Ou L, Wang W, Delyagina E, Lux C, et al. Targeted delivery of human VEGF gene via complexes of magnetic nanoparticle-adenoviral vectors enhanced cardiac regeneration. PLoS One. 2012; 7:e39490. PMID: 22844395.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Corrigendum: Enhanced bone morphogenic protein adenoviral gene delivery to bone marrow stromal cells using magnetic nanoparticle
- Comparison of Human Bone Marrow Stromal Cells with Fibroblasts in Cell Proliferation and Collagen Synthesis
- An Immune-compromised Method for Tooth Transplantation Using Adult Bone Marrow Stromal Cells and Embryonic Tooth Germ
- Bone Morphogenetic Protein Receptor in the Osteogenic Differentiation of Rat Bone Marrow Stromal Cells
- Generation and Characterization of 1H8 monoclonal antibody against human bone marrow stromal cells