Anat Cell Biol.
2010 Dec;43(4):284-293. 10.5115/acb.2010.43.4.284.
HOXB13 is co-localized with androgen receptor to suppress androgen-stimulated prostate-specific antigen expression
- Affiliations
-
- 1Department of Anatomy, Chonnam National University Medical School, Gwangju, Korea. chjung@chonnam.ac.kr
- 2Department of Urology, Chonnam National University Medical School, Gwangju, Korea.
- 3Research Institute of Medical Sciences, Chonnam National University, Gwangju, Korea.
- KMID: 1455342
- DOI: http://doi.org/10.5115/acb.2010.43.4.284
Abstract
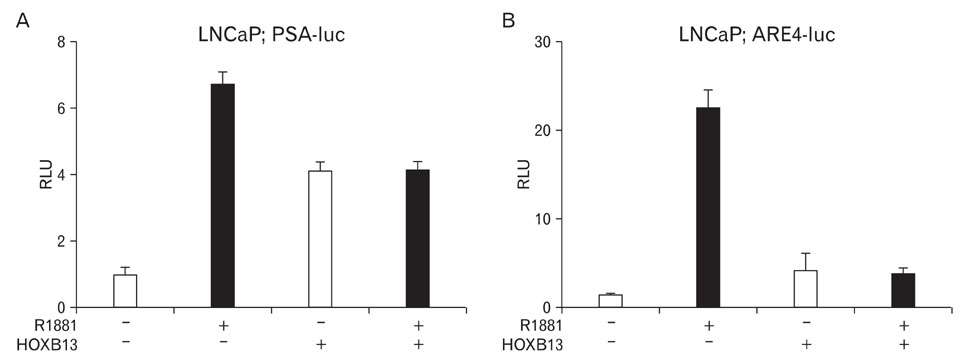
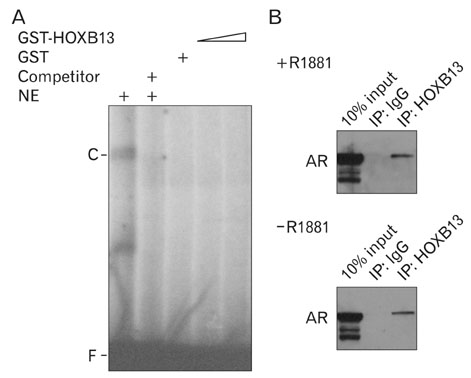
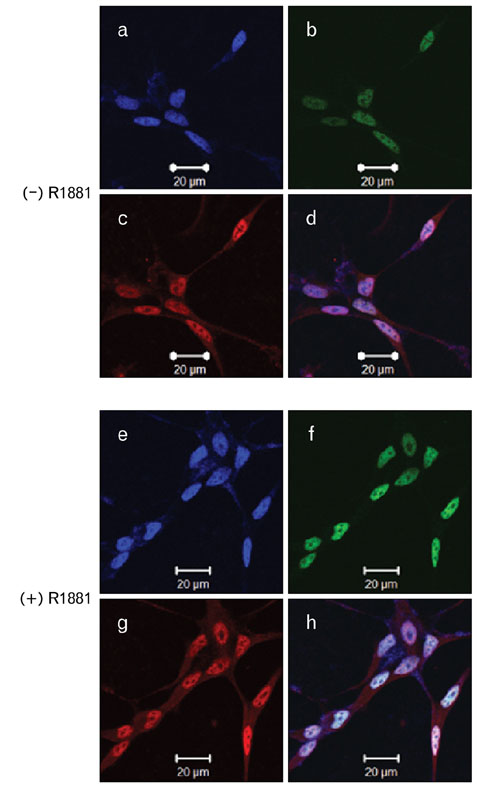
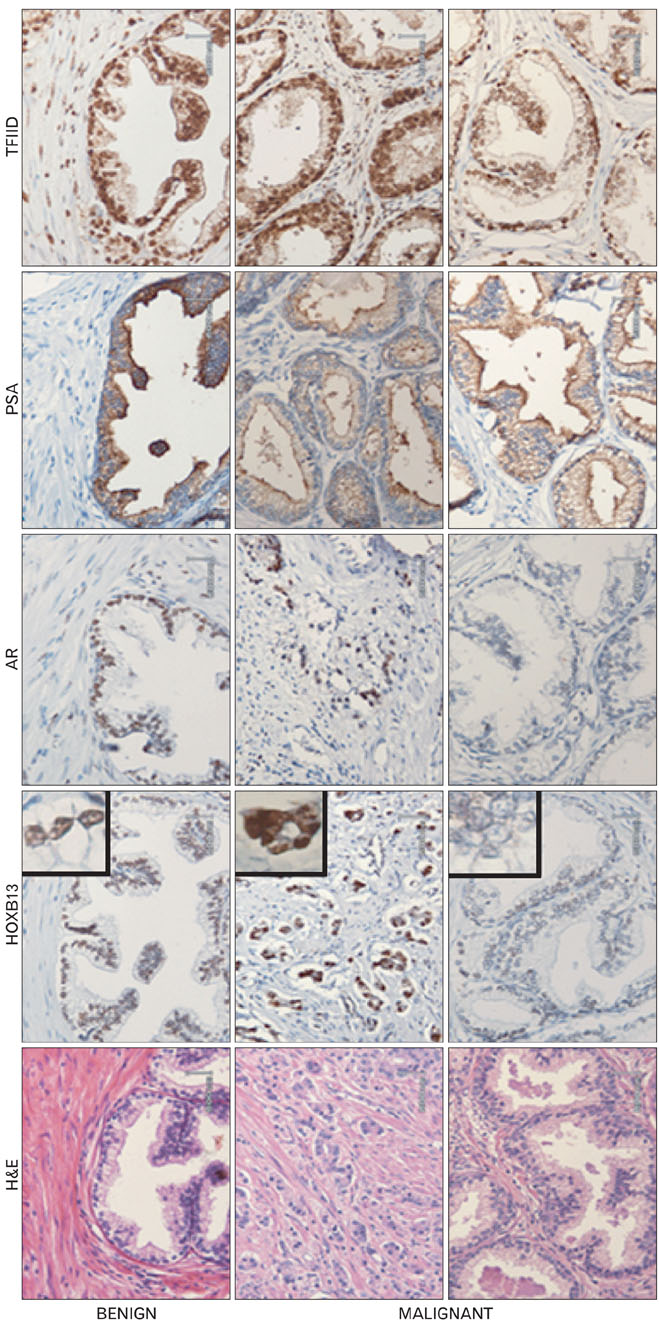
- During the prostate cancer (PCa) development and its progression into hormone independency, androgen receptor (AR) signals play a central role by triggering the regulation of target genes, including prostate-specific antigen. However, the regulation of these AR-mediated target genes is not fully understood. We have previously demonstrated a unique role of HOXB13 homeodomain protein as an AR repressor. Expression of HOXB13 was highly restricted to the prostate and its suppression dramatically increased hormone-activated AR transactivation, suggesting that prostate-specific HOXB13 was a highly potent transcriptional regulator. In this report, we demonstrated the action mechanism of HOXB13 as an AR repressor. HOXB13 suppressed androgen-stimulated AR activity by interacting with AR. HOXB13 did neither bind to AR responsive elements nor disturb nuclear translocation of AR in response to androgen. In PCa specimen, we also observed mutual expression pattern of HOXB13 and AR. These results suggest that HOXB13 not only serve as a DNA-bound transcription factor but play an important role as an AR-interacting repressor to modulate hormone-activated androgen receptor signals. Further extensive studies will uncover a novel mechanism for regulating AR-signaling pathway to lead to expose new role of HOXB13 as a non-DNA-binding transcriptional repressor.
Keyword
MeSH Terms
Figure
Reference
-
1. Catron KM, Zhang H, Marshall SC, Inostroza JA, Wilson JM, Abate C. Transcriptional repression by Msx-1 does not require homeodomain DNA-binding sites. Mol Cell Biol. 1995. 15:861–871.2. Chariot A, van Lint C, Chapelier M, Gielen J, Merville MP, Bours V. CBP and histone deacetylase inhibition enhance the transactivation potential of the HOXB7 homeodomain-containing protein. Oncogene. 1999. 18:4007–4014.3. Economides KD, Capecchi MR. Hoxb13 is required for normal differentiation and secretory function of the ventral prostate. Development. 2003. 130:2061–2069.4. Economides KD, Zeltser L, Capecchi MR. Hoxb13 mutations cause overgrowth of caudal spinal cord and tail vertebrae. Dev Biol. 2003. 256:317–330.5. Edwards S, Campbell C, Flohr P, et al. Expression analysis onto microarrays of randomly selected cDNA clones highlights HOXB13 as a marker of human prostate cancer. Br J Cancer. 2005. 92:376–381.6. Hood L, Heath JR, Phelps ME, Lin B. Systems biology and new technologies enable predictive and preventative medicine. Science. 2004. 306:640–643.7. Jung C, Kim RS, Lee SJ, Wang C, Jeng MH. HOXB13 homeodomain protein suppresses the growth of prostate cancer cells by the negative regulation of T-cell factor 4. Cancer Res. 2004a. 64:3046–3051.8. Jung C, Kim RS, Zhang HJ, Lee SJ, Jeng MH. HOXB13 induces growth suppression of prostate cancer cells as a repressor of hormone-activated androgen receptor signaling. Cancer Res. 2004b. 64:9185–9192.9. Kim YR, Oh KJ, Park RY, et al. HOXB13 promotes androgen independent growth of LNCaP prostate cancer cells by the activation of E2F signaling. Mol Cancer. 2010. 9:124.10. Lagorce-Pages C, Paraf F, Dubois S, Belghiti J, Fléjou JF. Expression of CD44 in premalignant and malignant Barrett's oesophagus. Histopathology. 1998. 32:7–14.11. Lee SJ, Kim HS, Yu R, et al. Novel prostate-specific promoter derived from PSA and PSMA enhancers. Mol Ther. 2002. 6:415–421.12. Lee SJ, Lee K, Yang X, et al. NFATc1 with AP-3 site binding specificity mediates gene expression of prostate-specific-membrane-antigen. J Mol Biol. 2003. 330:749–760.13. Levine M, Hoey T. Homeobox proteins as sequence-specific transcription factors. Cell. 1988. 55:537–540.14. Mann RS, Chan SK. Extra specificity from extradenticle: the partnership between HOX and PBX/EXD homeodomain proteins. Trends Genet. 1996. 12:258–262.15. Podlasek CA, Clemens JQ, Bushman W. Hoxa-13 gene mutation results in abnormal seminal vesicle and prostate development. J Urol. 1999a. 161:1655–1661.16. Podlasek CA, Duboule D, Bushman W. Male accessory sex organ morphogenesis is altered by loss of function of Hoxd-13. Dev Dyn. 1997. 208:454–465.17. Podlasek CA, Seo RM, Clemens JQ, Ma L, Maas RL, Bushman W. Hoxa-10 deficient male mice exhibit abnormal development of the accessory sex organs. Dev Dyn. 1999b. 214:1–12.18. Prins GS, Birch L, Habermann H, et al. Influence of neonatal estrogens on rat prostate development. Reprod Fertil Dev. 2001. 13:241–252.19. Raman V, Martensen SA, Reisman D, et al. Compromised HOXA5 function can limit p53 expression in human breast tumours. Nature. 2000a. 405:974–978.20. Raman V, Tamori A, Vali M, Zeller K, Korz D, Sukumar S. HOXA5 regulates expression of the progesterone receptor. J Biol Chem. 2000b. 275:26551–26555.21. Schnabel CA, Abate-Shen C. Repression by HoxA7 is mediated by the homeodomain and the modulatory action of its N-terminal-arm residues. Mol Cell Biol. 1996. 16:2678–2688.22. Shen W, Chrobak D, Krishnan K, Lawrence HJ, Largman C. HOXB6 protein is bound to CREB-binding protein and represses globin expression in a DNA binding-dependent, PBX interaction-independent process. J Biol Chem. 2004. 279:39895–39904.23. Shen WF, Chang CP, Rozenfeld S, et al. Hox homeodomain proteins exhibit selective complex stabilities with Pbx and DNA. Nucleic Acids Res. 1996. 24:898–906.24. Shen WF, Krishnan K, Lawrence HJ, Largman C. The HOX homeodomain proteins block CBP histone acetyltransferase activity. Mol Cell Biol. 2001. 21:7509–7522.25. Shen WF, Montgomery JC, Rozenfeld S, et al. AbdB-like Hox proteins stabilize DNA binding by the Meis1 homeodomain proteins. Mol Cell Biol. 1997. 17:6448–6458.26. Sreenath T, Orosz A, Fujita K, Bieberich CJ. Androgen-independent expression of hoxb-13 in the mouse prostate. Prostate. 1999. 41:203–207.27. Takahashi Y, Hamada J, Murakawa K, et al. Expression profiles of 39 HOX genes in normal human adult organs and anaplastic thyroid cancer cell lines by quantitative real-time RT-PCR system. Exp Cell Res. 2004. 293:144–153.28. Warot X, Fromental-Ramain C, Fraulob V, Chambon P, Dollé P. Gene dosage-dependent effects of the Hoxa-13 and Hoxd-13 mutations on morphogenesis of the terminal parts of the digestive and urogenital tracts. Development. 1997. 124:4781–4791.29. Wimmel A, Kogan E, Ramaswamy A, Schuermann M. Variant expression of CD44 in preneoplastic lesions of the lung. Cancer. 2001. 92:1231–1236.30. Zappavigna V, Sartori D, Mavilio F. Specificity of HOX protein function depends on DNA-protein and protein-protein interactions, both mediated by the homeo domain. Genes Dev. 1994. 8:732–744.31. Zeltser L, Desplan C, Heintz N. Hoxb-13: a new Hox gene in a distant region of the HOXB cluster maintains colinearity. Development. 1996. 122:2475–2484.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical Assay of Androgen Receptor in Prostate Cancer
- Differentiation Related Gene (Drg-1) as a Molecular Marker during the Treatment of in vitro Intermittent Androgen Deprivation in prostate Cancer
- Long-Lasting Antiandrogen Withdrawal Syndrome in Castration-Resistant Prostate Cancer: Three Cases With Complete Response
- Intermittent Androgen Deprivation with Goserelin and Flutamide for Prostate Cancer: a Pilot Study
- Role of Androgen Receptor in Prostate Cancer: A Review