Anat Cell Biol.
2011 Dec;44(4):331-336. 10.5115/acb.2011.44.4.331.
Increase in concentration of soluble HLA-G in high-quality embryos after intracytoplasmic sperm injection
- Affiliations
-
- 1Department of Anatomy, Shahid Beheshti Medical University, Velenjak, Tehran, Iran. hdr@sbmu.ac.ir
- 2Institute of Bioscience, UPM University, Serdang, Selangor, Malaysia.
- 3IVF Center, Taleghani Hospital, Iran.
- 4Cellular and Molecular Research, Shahid Beheshti Medical University, Velenjak, Iran.
- 5Medical University of Tehran, Kargar, Tehran, Iran.
- 6Reproductive Laboratory, Tohoko University, Sendai, Japan.
- KMID: 1447447
- DOI: http://doi.org/10.5115/acb.2011.44.4.331
Abstract
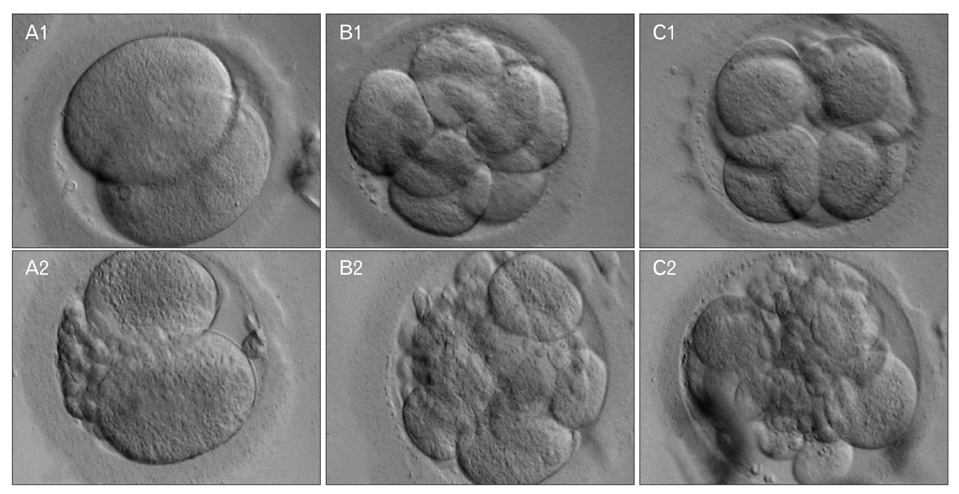
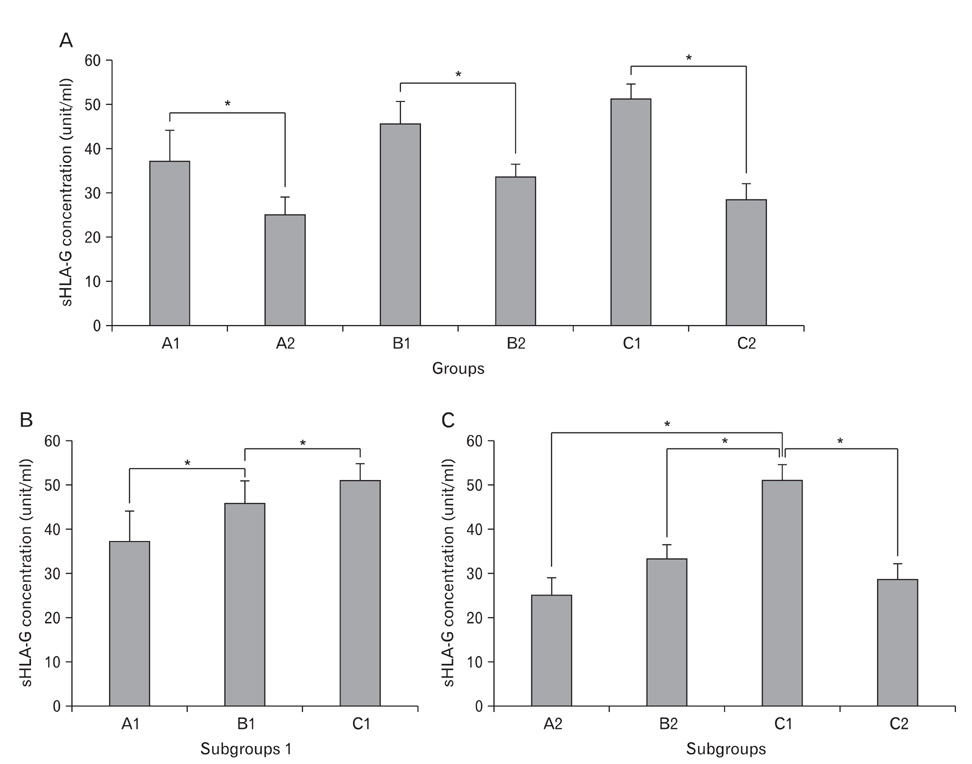
- Non-invasive methods are normally preferred to conventional invasive methods when selecting suitable embryos to improve pregnancy rates after assisted reproduction techniques. One of the most recognized non-invasive methods is to examine the supernatants of embryo culture media. Soluble human leukocyte antigen, class I, G (sHLA-G) antigen is a non-classical class I molecule that has been widely considered as a marker of pregnancy failure or implantation success. In the current study of some Iranian patients, we examined the concentration of sHLA-G at different time points after intracytoplasmic sperm injection and compared the rates to the morphology and quality of the selected embryos. We showed that the concentration of sHLA-G increases over time in high-quality embryos. We conclude that there is a positive relationship between morphology, quality, and sHLA-G concentration. We suggest that this relationship can be used to increase the chance of a successful pregnancy.
MeSH Terms
Figure
Reference
-
1. Noci I, Fuzzi B, Rizzo R, Melchiorri L, Criscuoli L, Dabizzi S, Biagiotti R, Pellegrini S, Menicucci A, Baricordi OR. Embryonic soluble HLA-G as a marker of developmental potential in embryos. Hum Reprod. 2005. 20:138–146.2. Lessey BA. Embryo quality and endometrial receptivity: lessons learned from the ART experience. J Assist Reprod Genet. 1998. 15:173–176.3. Fisch JD, Rodriguez H, Ross R, Overby G, Sher G. The Graduated Embryo Score (GES) predicts blastocyst formation and pregnancy rate from cleavage-stage embryos. Hum Reprod. 2001. 16:1970–1975.4. Suzumori N, Sugiura-Ogasawara M. Genetic factors as a cause of miscarriage. Curr Med Chem. 2010. 17:3431–3437.5. Rebmann V, Switala M, Eue I, Grosse-Wilde H. Soluble HLA-G is an independent factor for the prediction of pregnancy outcome after ART: a German multi-centre study. Hum Reprod. 2010. 25:1691–1698.6. Ellis SA, Palmer MS, McMichael AJ. Human trophoblast and the choriocarcinoma cell line BeWo express a truncated HLA Class I molecule. J Immunol. 1990. 144:731–735.7. Paul P, Cabestre FA, Ibrahim EC, Lefebvre S, Khalil-Daher I, Vazeux G, Quiles RM, Bermond F, Dausset J, Carosella ED. Identification of HLA-G7 as a new splice variant of the HLA-G mRNA and expression of soluble HLA-G5, -G6, and -G7 transcripts in human transfected cells. Hum Immunol. 2000. 61:1138–1149.8. Rebmann V, Switala M, Eue I, Schwahn E, Merzenich M, Grosse-Wilde H. Rapid evaluation of soluble HLA-G levels in supernatants of in vitro fertilized embryos. Hum Immunol. 2007. 68:251–258.9. Sher G, Keskintepe L, Nouriani M, Roussev R, Batzofin J. Expression of sHLA-G in supernatants of individually cultured 46-h embryos: a potentially valuable indicator of 'embryo competency' and IVF outcome. Reprod Biomed Online. 2004. 9:74–78.10. Hunt JS, Petroff MG, McIntire RH, Ober C. HLA-G and immune tolerance in pregnancy. FASEB J. 2005. 19:681–693.11. Hviid TV, Hylenius S, Lindhard A, Christiansen OB. Association between human leukocyte antigen-G genotype and success of in vitro fertilization and pregnancy outcome. Tissue Antigens. 2004. 64:66–69.12. Moreau P, Paul P, Rouas-Freiss N, Kirszenbaum M, Dausset J, Carosella ED. Molecular and immunologic aspects of the nonclassical HLA class I antigen HLA-G: evidence for an important role in the maternal tolerance of the fetal allograft. Am J Reprod Immunol. 1998. 40:136–144.13. Ng ST, Chang TH, Wu TC. Prediction of the rates of fertilization, cleavage, and pregnancy success by cumulus-coronal morphology in an in vitro fertilization program. Fertil Steril. 1999. 72:412–417.14. Choudhury SR, Knapp LA. Human reproductive failure II: immunogenetic and interacting factors. Hum Reprod Update. 2001. 7:135–160.15. Menicucci A, Noci I, Fuzzi B, Criscuoli L, Scarselli G, Baricordi O, Mattiuz PL. Non-classic sHLA class I in human oocyte culture medium. Hum Immunol. 1999. 60:1054–1057.16. Bamberger AM, Jenatschke S, Schulte HM, Löning T, Bamberger MC. Leukemia inhibitory factor (LIF) stimulates the human HLA-G promoter in JEG3 choriocarcinoma cells. J Clin Endocrinol Metab. 2000. 85:3932–3936.17. Margreiter M, Weghofer A, Kogosowski A, Mahmoud KZ, Feichtinger W. A prospective randomized multicenter study to evaluate the best day for embryo transfer: does the outcome justify prolonged embryo culture? J Assist Reprod Genet. 2003. 20:91–94.18. Riteau B, Rouas-Freiss N, Menier C, Paul P, Dausset J, Carosella ED. HLA-G2, -G3, and -G4 isoforms expressed as nonmature cell surface glycoproteins inhibit NK and antigen-specific CTL cytolysis. J Immunol. 2001. 166:5018–5026.19. Kotze DJ, Hansen P, Keskintepe L, Snowden E, Sher G, Kruger T. Embryo selection criteria based on morphology VERSUS the expression of a biochemical marker (sHLA-G) and a graduated embryo score: prediction of pregnancy outcome. J Assist Reprod Genet. 2010. 27:309–316.20. Alinejad Z, Jafari Shakib R, Forghan-Parast K, Zahiri Z, Sadri H, Nagafi F, Roushan Z. In vitro fertilized embryos do not secrete detectable HLA-G on day two. Iran J Immunol. 2009. 6:195–201.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Pregnancy from Embryos following ICSI with Frozen-Thawed Testicular Sperms
- Twin Pregnancy and Delivery After Intracytoplasmic Sperm Injection Followed by Calcium Ionophore with Spermatozoa from a Globozoospermic Man: A Case Report
- A Case of Pregnancy from Cryopreserved Embryos following ICSI with Frozen-Thawed Epididymal Sperms
- High mRNA expression of GABA receptors in human sperm with oligoasthenoteratozoospermia and teratozoospermia and its association with sperm parameters and intracytoplasmic sperm injection outcomes
- Physiological intracytoplasmic sperm injection does not improve the quality of embryos: A cross-sectional investigation on sibling oocytes