J Korean Surg Soc.
2012 Dec;83(6):343-351. 10.4174/jkss.2012.83.6.343.
Chronic allograft injury by subclinical borderline change: evidence from serial protocol biopsies in kidney transplantation
- Affiliations
-
- 1Department of Surgery, Seoul National University College of Medicine, Seoul, Korea. jwhamd@snu.ac.kr
- 2Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 4Transplantation Research Institute, Seoul National University Medical Research Center, Seoul, Korea.
- KMID: 1437529
- DOI: http://doi.org/10.4174/jkss.2012.83.6.343
Abstract
- PURPOSE
This study investigated the impact of subclinical borderline changes on the development of chronic allograft injury in patients using a modern immunosuppression protocol.
METHODS
Seventy patients with stable renal allograft function and who underwent protocol biopsies at implantation, 10 days and 1 year after transplantation were included and classified based on biopsy findings at day 10. The no rejection (NR) group included 33 patients with no acute rejection. The treatment (Tx) group included 21 patients with borderline changes following steroid pulse therapy, and the nontreatment (NTx) group included 16 patients with borderline changes nontreated.
RESULTS
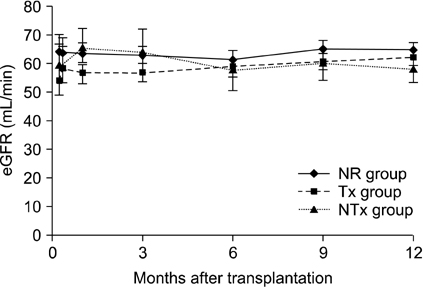
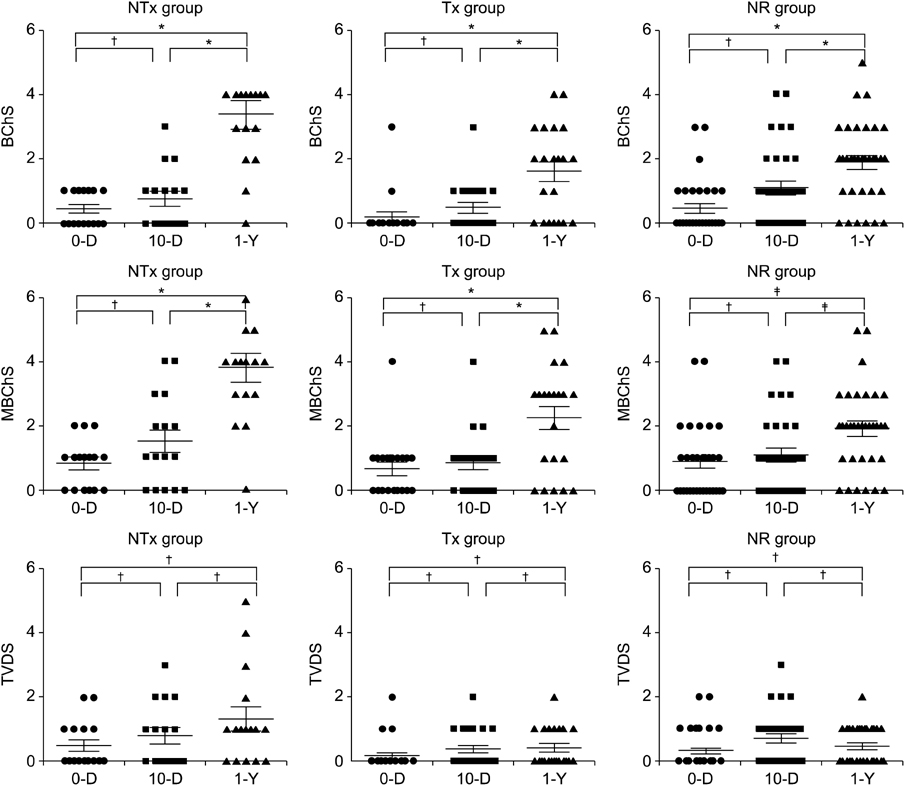
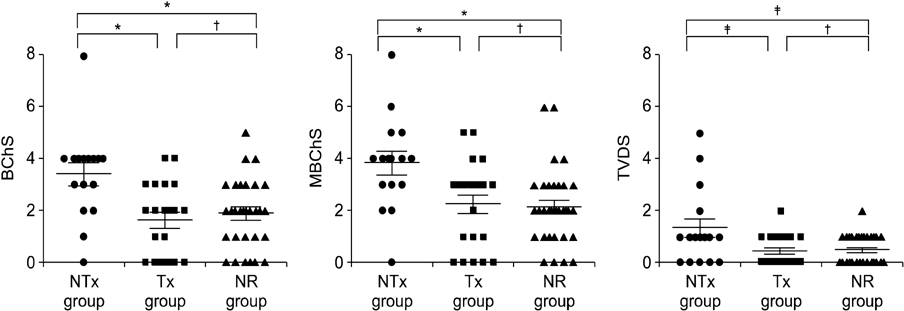
The Banff Chronicity Score (BChS) and modified BChS (MBChS) were not different among the three groups at implantation (P = 0.48) or on day 10 (P = 0.96). Surprisingly, the NTx group had more prominent chronic scores at the 1-year biopsy, including BChS (3.07 +/- 1.33, P = 0.005) and MBChS (3.14 +/- 1.41, P = 0.008) than those in the Tx and NR group, and deterioration of BChS was more noticeable in the NTx group (P = 0.037), although renal function was stable (P = 0.66). No difference in chronic injury scores was observed between the Tx and NR groups at the 1-year biopsy.
CONCLUSION
Subclinical borderline changes can be a risk factor for chronic allograft injury and should be considered for antirejection therapy.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Flat Pattern Peaks of Tacrolimus Absorption and Associated Pharmacogenomic Variants in Kidney Transplantation Recipients
Suh Min Kim, Younggyun Lim, Sangil Min, Byung-Joo Min, Myung-Eui Seo, Kye Hwa Lee, Ju Han Kim, Jongwon Ha
J Korean Med Sci. 2022;37(5):e33. doi: 10.3346/jkms.2022.37.e33.
Reference
-
1. Chapman JR, O'Connell PJ, Nankivell BJ. Chronic renal allograft dysfunction. J Am Soc Nephrol. 2005. 16:3015–3026.2. Miyagi M, Ishikawa Y, Mizuiri S, Aikawa A, Ohara T, Hasegawa A. Significance of subclinical rejection in early renal allograft biopsies for chronic allograft dysfunction. Clin Transplant. 2005. 19:456–465.3. Legendre C, Thervet E, Skhiri H, Mamzer-Bruneel MF, Cantarovich F, Noel LH, et al. Histologic features of chronic allograft nephropathy revealed by protocol biopsies in kidney transplant recipients. Transplantation. 1998. 65:1506–1509.4. Furness PN, Kirkpatrick U, Taub N, Davies DR, Solez K. A UK-wide trial of the Banff classification of renal transplant pathology in routine diagnostic practice. Nephrol Dial Transplant. 1997. 12:995–1000.5. Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999. 55:713–723.6. Solez K, Axelsen RA, Benediktsson H, Burdick JF, Cohen AH, Colvin RB, et al. International standardization of criteria for the histologic diagnosis of renal allograft rejection: the Banff working classification of kidney transplant pathology. Kidney Int. 1993. 44:411–422.7. Nankivell BJ, Chapman JR. The significance of subclinical rejection and the value of protocol biopsies. Am J Transplant. 2006. 6:2006–2012.8. Saad R, Gritsch HA, Shapiro R, Jordan M, Vivas C, Scantlebury V, et al. Clinical significance of renal allograft biopsies with "borderline changes," as defined in the Banff Schema. Transplantation. 1997. 64:992–995.9. Meehan SM, Siegel CT, Aronson AJ, Bartosh SM, Thistlethwaite JR, Woodle ES, et al. The relationship of untreated borderline infiltrates by the Banff criteria to acute rejection in renal allograft biopsies. J Am Soc Nephrol. 1999. 10:1806–1814.10. Schweitzer EJ, Drachenberg CB, Anderson L, Papadimetriou JC, Kuo PC, Johnson LB, et al. Significance of the Banff borderline biopsy. Am J Kidney Dis. 1996. 28:585–588.11. Min SI, Yun IJ, Kang JM, Park YJ, Min SK, Ahn C, et al. Moderate-to-severe early-onset hyperuricaemia: a prognostic marker of long-term kidney transplant outcome. Nephrol Dial Transplant. 2009. 24:2584–2590.12. Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008. 8:753–760.13. Racusen LC, Halloran PF, Solez K. Banff 2003 meeting report: new diagnostic insights and standards. Am J Transplant. 2004. 4:1562–1566.14. Kambham N, Nagarajan S, Shah S, Li L, Salvatierra O, Sarwal MM. A novel, semiquantitative, clinically correlated calcineurin inhibitor toxicity score for renal allograft biopsies. Clin J Am Soc Nephrol. 2007. 2:135–142.15. Ekberg H, Tedesco-Silva H, Demirbas A, Vitko S, Nashan B, Gurkan A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007. 357:2562–2575.16. Seron D, Moreso F, Bover J, Condom E, Gil-Vernet S, Canas C, et al. Early protocol renal allograft biopsies and graft outcome. Kidney Int. 1997. 51:310–316.17. Roberts IS, Reddy S, Russell C, Davies DR, Friend PJ, Handa AI, et al. Subclinical rejection and borderline changes in early protocol biopsy specimens after renal transplantation. Transplantation. 2004. 77:1194–1198.18. Heilman RL, Devarapalli Y, Chakkera HA, Mekeel KL, Moss AA, Mulligan DC, et al. Impact of subclinical inflammation on the development of interstitial fibrosis and tubular atrophy in kidney transplant recipients. Am J Transplant. 2010. 10:563–570.19. Thierry A, Thervet E, Vuiblet V, Goujon JM, Machet MC, Noel LH, et al. Long-term impact of subclinical inflammation diagnosed by protocol biopsy one year after renal transplantation. Am J Transplant. 2011. 11:2153–2161.20. Henderson LK, Nankivell BJ, Chapman JR. Surveillance protocol kidney transplant biopsies: their evolving role in clinical practice. Am J Transplant. 2011. 11:1570–1575.21. Rush D, Nickerson P, Gough J, McKenna R, Grimm P, Cheang M, et al. Beneficial effects of treatment of early subclinical rejection: a randomized study. J Am Soc Nephrol. 1998. 9:2129–2134.22. Gray D, Shepherd H, Daar A, Oliver DO, Morris PJ. Oral versus intravenous high-dose steroid treatment of renal allograft rejection. The big shot or not? Lancet. 1978. 1:117–118.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of steroid pulse therapy for the reduction of acute rejection episode in subclinical borderline changes: an open-label, randomized clinical trial
- The effect of steroid pulse therapy for the reduction of acute rejection episode in subclinical borderline changes: an open-label, randomized clinical trial
- Feasibility and safety of 2-week protocol biopsy after kidney transplantation
- Current safety and outcomes of kidney allograft biopsy
- The protective role of protocol biopsy against chronic kidney disease progression in kidney transplantation