Lab Anim Res.
2012 Sep;28(3):217-221. 10.5625/lar.2012.28.3.217.
Acute gastrointestinal dilation in laboratory rhesus monkeys in the Korea National Primate Research Center
- Affiliations
-
- 1The National Primate Research Center, Korea Research Institute of Bioscience and Biotechnology, Ochang, Korea. changkt@kribb.re.kr
- 2University of Science and Technology, Daejeon, Korea.
- 3College of Veterinary Medicine, Kangwon National University, Chuncheon, Korea.
- KMID: 1436728
- DOI: http://doi.org/10.5625/lar.2012.28.3.217
Abstract
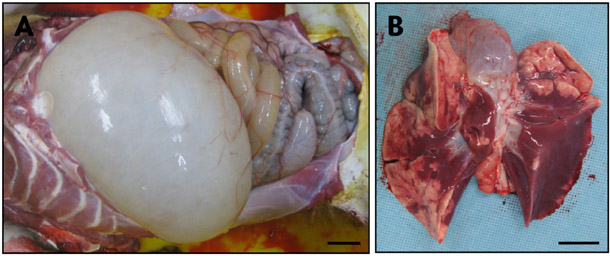
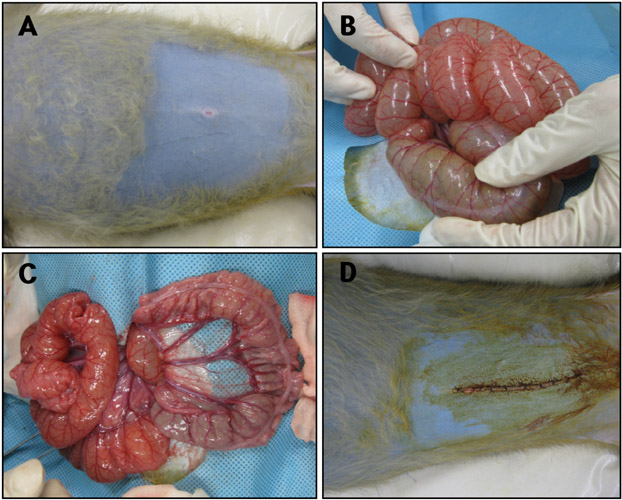
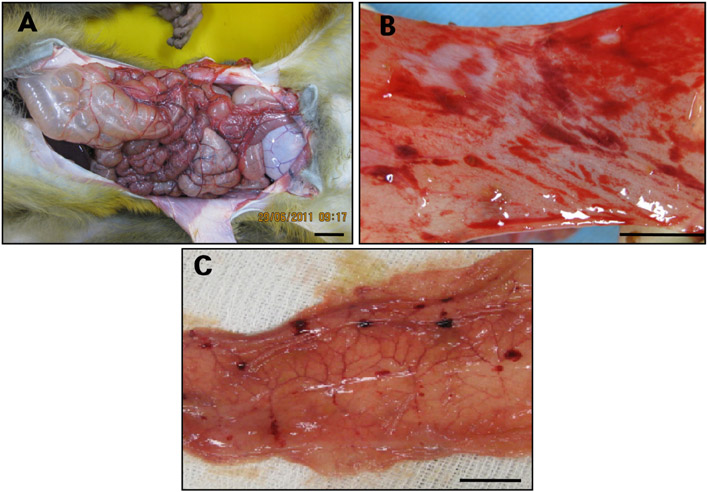
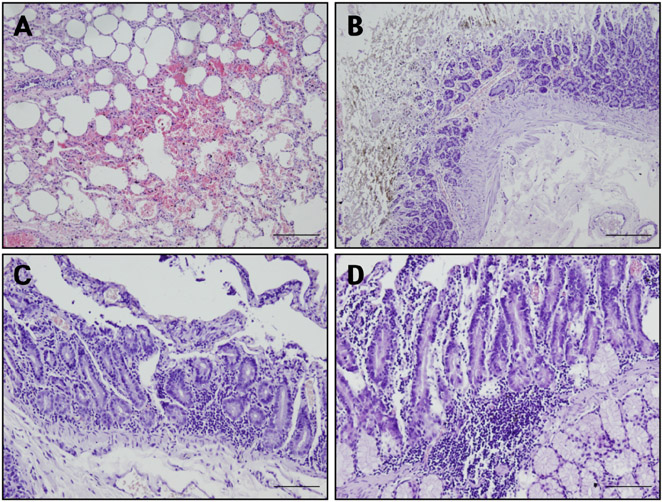
- Acute gastrointestinal dilation is a medical condition in which the stomach and intestine become overstretched by excessive gas content. In laboratory monkeys, cases of bloating involving gastrointestinal dilation are rarely seen, and the cause thereof is not clearly defined. Two rhesus monkeys in the Korea National Primate Research Center were found to suffer from acute gastrointestinal dilation. One of the monkeys showed severe gastric bloating after recovering from general anesthesia with isoflurane, where after it died suddenly. During necropsy, severe congestion of the lung was observed. The other monkey showed gastrointestinal dilation and died after treatment. During necropsy, severe dilation of the large intestine was observed. Severe congestion was detected in small and large intestines. Histopathologically, erythrocytes were found to fill the alveoli and alveolar capillaries of the lung. In stomach, epithelial cells were found to be sloughed from the mucosal layer, and erythrocytes were found to fill the blood vessels of the submucosal and mucosal layers. In small and large intestines, epithelial cells were also found to be sloughed from the mucosal layer, and inflammatory cells were found to have infiltrated in the submucosa (only large intestine) and mucosa. Microbiologically, Enterococcus faecalis and the pathogenic Staphylococcus haemolyticus, which do not form gas in the gastrointestinal tract, were detected in the gastrointestinal contents of both monkeys. These results suggest that the cause of the acute gastrointestinal dilation in these monkeys was not infection by gas-forming bacteria, but rather multiple factors such as diet, anesthesia, and excessive water consumption.
MeSH Terms
-
Anesthesia
Anesthesia, General
Bacteria
Blood Vessels
Capillaries
Diet
Drinking
Enterococcus faecalis
Epithelial Cells
Erythrocytes
Estrogens, Conjugated (USP)
Gastrointestinal Contents
Gastrointestinal Tract
Haplorhini
Intestine, Large
Intestines
Isoflurane
Korea
Lung
Macaca mulatta
Mucous Membrane
Primates
Staphylococcus haemolyticus
Stomach
Estrogens, Conjugated (USP)
Isoflurane
Figure
Reference
-
1. Pond CL, Newcomer CE, Anver MR. Acute gastric dilatation in nonhuman primates: review and case studies. Vet Pathol Suppl. 1982. 7:126–133.2. Min BR, Pinchak WE, Anderson RC, Hume ME. In vitro bacterial growth and in vivo ruminal microbiota populations associated with bloat in steers grazing wheat forage. J Anim Sci. 2006. 84(10):2873–2882.3. Soave OA. Observations on acute gastric dilatation in nonhuman primates. Lab Anim Sci. 1978. 28(3):331–334.4. Elwell MR, DePaoli A. Gastric dilatation and volvulus in a squirrel monkey. J Am Vet Med Assoc. 1978. 173(9):1235–1236.5. Smith AW, Casey HW, LaCroix JT, Johnson DK. Acute bloat syndrome (gastric dilatation) in Macaca mulatta. J Am Vet Med Assoc. 1969. 155(7):1241–1244.6. Weinstein MP, Mirrett S, Van Pelt L, McKinnon M, Zimmer BL, Kloos W, Reller LB. Clinical importance of identifying coagulase-negative staphylococci isolated from blood cultures: evaluation of MicroScan Rapid and Dried Overnight Gram-Positive panels versus a conventional reference method. J Clin Microbiol. 1998. 36(7):2089–2092.7. Fischetti A, Novick RP, Ferretti JJ, Portnoy DA, Rood JI, Lina G, Etienne J, Vandenesch F. Biology and pathogenicity of staphylococci other than Staphylococcus aureus and Staphylococcus epidermidis. Gram-positive pathogens. 2000. Washington, DC: ASM Press;450–462.8. Falcone M, Giannella M, Raponi G, Mancini C, Venditti M. Teicoplanin use and emergence of Staphylococcus haemolyticus: is there a link? Clin Microbiol Infect. 2006. 12(1):96–97.9. Poyart C, Quesne G, Boumaila C, Trieu-Cuot P. Rapid and accurate species-level identification of coagulase-negative staphylococci by using the sodA gene as a target. J Clin Microbiol. 2001. 39(12):4296–4301.10. Viale P, Stefani S. Vascular catheter-associated infections: a microbiological and therapeutic update. J Chemother. 2006. 18(3):235–249.11. de Allori MC, Jure MA, Romero C, de Castillo ME. Antimicrobial resistance and production of biofilms in clinical isolates of coagulase-negative Staphylococcus strains. Biol Pharm Bull. 2006. 29(8):1592–1596.12. Ryan KJ, Ray CG. Sherris Medical Microbiology. 2004. 4th ed. McGraw Hill;294–295.13. Murray BE. The life and times of the Enterococcus. Clin Microbiol Rev. 1990. 3(1):46–65.14. Byrne JJ, Cahill JM. Acute gastric dilation. Am J Surg. 1961. 101:301–309.15. Danhof IE. The clinical gas syndromes: a pathophysiologic approach. Ann N Y Acad Sci. 1968. 150(1):127–140.16. Newton WM, Beamer PD, Rhoades HE. Acute bloat syndrome in stumptailed macaques (Macaca arctoides): a report of four cases. Lab Anim Sci. 1971. 21(2):193–196.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reference values of hematological and biochemical parameters in young-adult cynomolgus monkey (Macaca fascicularis) and rhesus monkey (Macaca mulatta) anesthetized with ketamine hydrochloride
- Heavy metal concentrations in hair of newly imported China-origin rhesus macaques (Macaca mulatta)
- Suppurative bite wound by repetitive aggression of dominance hierarchy during group housing in rhesus monkeys
- Intratracheal inoculation of human varicella zoster virus (VZV; MAV strain) vaccine successfully induced VZV IgG antibodies in rhesus monkeys
- Evaluation of the Differences of Myocardial Fibers between Acute and Chronic Myocardial Infarction: Application of Diffusion Tensor Magnetic Resonance Imaging in a Rhesus Monkey Model