Lab Anim Res.
2013 Jun;29(2):103-112. 10.5625/lar.2013.29.2.103.
Therapeutic effects of fermented soycrud on phenotypes of atopic dermatitis induced by phthalic anhydride
- Affiliations
-
- 1Department of Biomaterials Science, College of Natural Resources & Life Science, Pusan National University, Miryang, Korea. dyhwang@pusan.ac.kr
- 2Department of Food Science & Technology, College of Natural Resources & Life Science, Pusan National University, Miryang, Korea.
- KMID: 1431343
- DOI: http://doi.org/10.5625/lar.2013.29.2.103
Abstract
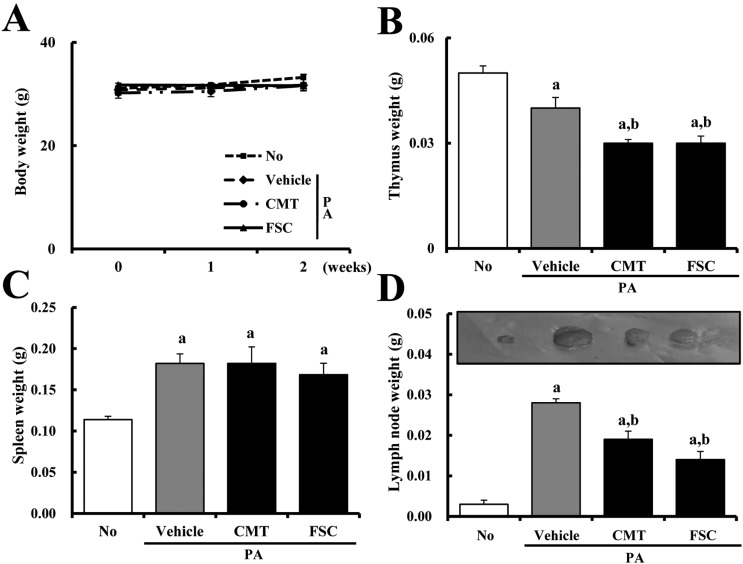
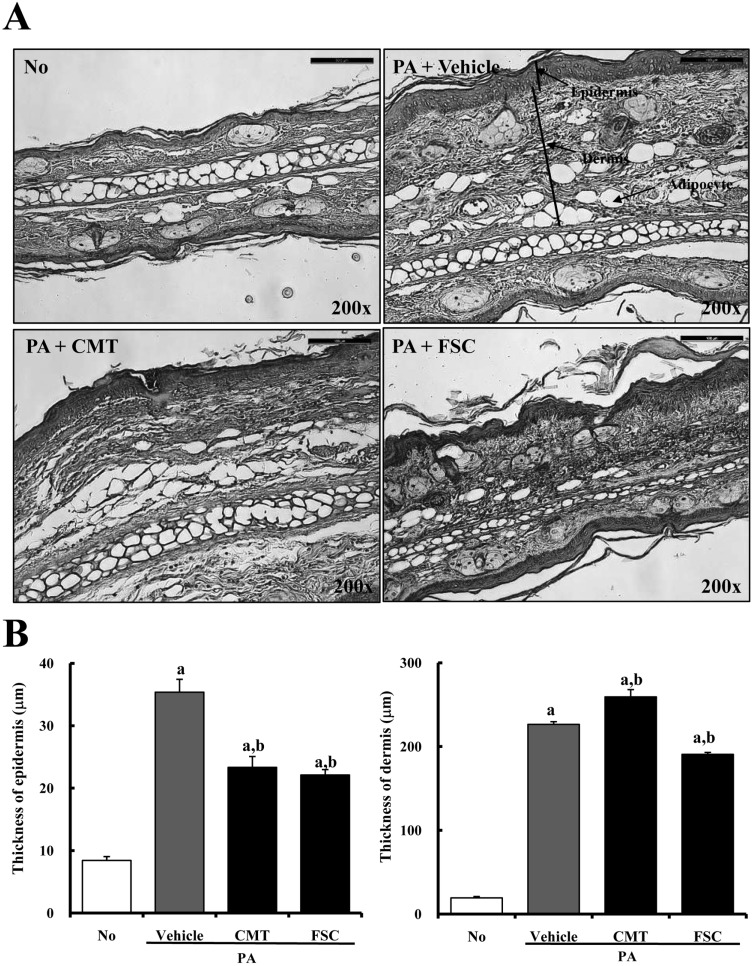
- Atopic dermatitis (AD), which is known as the most common pruritic skin disease, is caused by epidermal barrier dysfunction, allergies, microwave radiation, histamine intolerance, and genetic defects. To investigate the therapeutic effects of fermented soycrud (FSC) on AD pathology, alteration of AD phenotypes induced by phthalic anhydride (PA) treatment was assessed by ear thickness analysis, measurement of immune-related organ weights, ELISA, and histological and pathological analyses of ICR mice after FSC treatment for 2 weeks. Except for water content, the concentrations of most major components were lower in FSC compared to common tofu (CMT). Thymus and lymph node weights were significantly reduced in ICR mice treated with PA+CMT or PA+FSC, whereas spleen and body weights were maintained. Elevation of ear thickness induced by PA treatment was rapidly diminished in the CMT- and FSC-treated groups, although there was no significant difference between the two groups. Furthermore, significant reduction of epidermal thickness was detected in both the PA+CMT- and PA+FSC-treated groups. However, IgE concentration and dermal thickness were reduced only by PA+FSC treatment, whereas PA+CMT treatment maintained levels comparable to PA+vehicle treatment. The number of infiltrated mast cells was higher in the PA+vehicle-treated group compared to the untreated control. Following CMT or FSC treatment, mast cell infiltration was slightly reduced, although the CMT-treated group showed greater cell numbers. These results indicate that FSC may significantly relieve the phenotypes of AD induced by PA treatment and should be considered as a potential candidate for AD therapy.
MeSH Terms
-
Animals
Body Weight
Cell Count
Dermatitis, Atopic
Ear
Enzyme-Linked Immunosorbent Assay
Histamine
Hypersensitivity
Immunoglobulin E
Lymph Nodes
Mast Cells
Mice
Mice, Inbred ICR
Microwaves
Organ Size
Phenotype
Phthalic Anhydrides
Skin Diseases
Soy Foods
Spleen
Thymus Gland
Water
Weights and Measures
Histamine
Immunoglobulin E
Phthalic Anhydrides
Water
Figure
Reference
-
1. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980; 92:44–47.3. Kay AB. Overview of 'allergy and allergic diseases: with a view to the future'. Br Med Bull. 2000; 56(4):843–864. PMID: 11359624.
Article4. Kay AB. Allergy and allergic diseases. First of two parts. N Engl J Med. 2001; 344(1):30–37. PMID: 11136958.5. Grammatikos AP. The genetic and environmental basis of atopic diseases. Ann Med. 2008; 40(7):482–495. PMID: 18608118.
Article6. Levy ML. Atopic dermatitis: understanding the disease and its management. Curr Med Res Opin. 2007; 23(12):3091–3103. PMID: 17971284.
Article7. Simpson EL. Atopic dermatitis: a review of topical treatment options. Curr Med Res Opin. 2010; 26(3):633–640. PMID: 20070141.
Article8. Pariser D. Topical corticosteroids and topical calcineurin inhibitors in the treatment of atopic dermatitis: focus on percutaneous absorption. Am J Ther. 2009; 16(3):264–273. PMID: 19262357.
Article9. Draelos ZD. Use of topical corticosteroids and topical calcineurin inhibitors for the treatment of atopic dermatitis in thin and sensitive skin areas. Curr Med Res Opin. 2008; 24(4):985–994. PMID: 18284804.
Article10. Cai TD, Chang KC. Dry tofu characteristics affected by soymilk solid content and coagulation time. J Food Qual. 1997; 20:391–402.
Article11. Cai TD, Chang KC. Characteristics of production-scale tofu as affected by soymilk coagulation method: propeller blade size, mixing time and coagulant concentration. Food Res Intl. 1998; 31(4):289–295.
Article12. Toda T, Tamura J, Okuhira T. Isoflavone content in commercial soybean food. Foods Food Ingredients J. 1997; 172:83–88.13. Ashton EL, Dalais FS, Ball MJ. Effect of meat replacement by tofu on CHD risk factors including copper induced LDL oxidation. J Am Coll Nutr. 2000; 19(6):761–767. PMID: 11194529.
Article14. Oboh G. Coagulants modulate the antioxidant properties and hypocholesterolemic effect of tofu (Curdled soymilk). Asian J Biochem. 2006; 1(1):57–66.
Article15. Choi YS, Lee SY. Cholesterol-lowering effects of soybean products (Curd or Curd residue) in rats. J Korean Soc Food Nutr. 1993; 22(6):673–677.16. Sonoda T, Nagata Y, Mori M, Miyanaga N, Takashima N, Okumura K, Goto K, Naito S, Fujimoto K, Hirao Y, Takahashi A, Tsukamoto T, Fujioka T, Akaza H. A case-control study of diet and prostate cancer in Japan: possible protective effect of traditional Japanese diet. Cancer Sci. 2004; 95(3):238–242. PMID: 15016323.
Article17. Wu AH, Ziegler RG, Horn-Ross PL, Nomura AM, West DW, Kolonel LN, Rosenthal JF, Hoover RN, Pike MC. Tofu and risk of breast cancer in Asian-Americans. Cancer Epidemiol Biomarkers Prev. 1996; 5(11):901–906. PMID: 8922298.18. Lee JK, Park BJ, Yoo KY, Ahn YO. Dietary factors and stomach cancer: a case-control study in Korea. Int J Epidemiol. 1995; 24(1):33–41. PMID: 7797354.
Article19. Liu CY, Hsu YH, Wu MT, Pan PC, Ho CK, Su L, Xu X, Li Y, Christiani DC. Kaohsiung Leukemia Research Group. Cured meat, vegetables, and bean-curd foods in relation to childhood acute leukemia risk: a population based case-control study. BMC Cancer. 2009; 9:9–15. PMID: 19134212.
Article20. Liu J, Chang SK, Wiesenborn D. Antioxidant properties of soybean isoflavone extract and tofu in vitro and in vivo. J Agric Food Chem. 2005; 53(6):2333–2340. PMID: 15769177.21. Dubois M, Gillers KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugar and related substance. Anal Chem. 1956; 28:350–352.22. Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959; 31(3):426–428.
Article23. Kim HJ, Kim J, Kim SJ, Lee SH, Park YS, Park BK, Kim BS, Kim SK, Cho SD, Jung JW, Nam JS, Choi CS, Jung JY. Anti-inflammatory effect of quercetin on picryl chloride-induced contact dermatitis in BALB/c mice. Lab Anim Res. 2010; 26(1):7–13.
Article24. Hayashi M, Higashi K, Kato H, Kaneko H. Assessment of preferential Th1 or Th2 induction by low-molecular-weight compounds using a reverse transcription-polymerase chain reaction method: comparison of two mouse strains, C57BL/6 and BALB/c. Toxicol Appl Pharmacol. 2001; 177(1):38–45. PMID: 11708898.
Article25. Ban M, Hettich D. Effect of Th2 cytokine antagonist treatments on chemical-induced allergic response in mice. J Appl Toxicol. 2005; 25(3):239–247. PMID: 15895478.
Article26. Kimber I, Dearman RJ. The mechanisms and evaluation of chemically induced allergy. Toxicol Lett. 1992; 64-65:79–84. PMID: 1471238.
Article27. Bae CJ, Shim SB, Jee SW, Lee SH, Kim MR, Lee JW, Lee CK, Hwang DY. IL-6, VEGF, KC, and RNATES are a major cause of a high irritant dermatitis to phthalic anhydride in C57BL/6 inbred mice. Allergol Int. 2010; 59(4):389–397. PMID: 20864798.28. Bae CJ, Lee JW, Bae HS, Shim SB, Jee SW, Lee SH, Lee CK, Hong JT, Hwang DY. Detection of allergenic compounds using an IL-4/Luciferase/CNS-1 transgenic mice model. Toxicol Sci. 2011; 120(2):349–359. PMID: 21252390.
Article29. Mori T, Tanimoto Y, Ota M, Masakado T, Kitamoto S, Saito K, Isobe N, Kaneko H. Comparison of cytokine profiles in bronchoalveolar lavage fluid of mice exposed to respiratory and contact sensitizers. J Toxicol Sci. 2012; 37(2):337–343. PMID: 22467024.
Article30. Fukuyama T, Tajima Y, Ueda H, Hayashi K, Shutoh Y, Harada T, Kosaka T. A method for measuring mouse respiratory allergic reaction to low-dose chemical exposure to allergens: an environmental chemical of uncertain allergenicity, a typical contact allergen and a non-sensitizing irritant. Toxicol Lett. 2010; 195(1):35–43. PMID: 20219652.
Article31. Miyake Y, Sasaki S, Ohya Y, Miyamoto S, Matsunaga I, Yoshida T, Hirota Y, Oda H. Soy, isoflavones, and prevalence of allergic rhinitis in Japanese women: the osaka maternal and child health study. J Allergy Clin Immunol. 2005; 115(6):1176–1183. PMID: 15940131.
Article32. Kobayashi M. Immunological functions of soy sauce: hypoallergenicity and antiallergic activity of soy sauce. J Biosci Bioeng. 2005; 100(2):144–151. PMID: 16198255.
Article33. Matsuda A, Tanaka A, Pan W, Okamoto N, Oida K, Kingyo N, Amagai Y, Xia Y, Jang H, Nishikawa S, Kajiwara N, Ahn G, Ohmori K, Matsuda H. Supplementation of the fermented soy product ImmuBalance™ effectively reduces itching behavior of atopic NC/Tnd mice. J Dermatol Sci. 2012; 67(2):130–139. PMID: 22748506.
Article34. Masuda Y, Takahashi T, Yoshida K, Nishitani Y, Mizuno M, Mizoguchi H. Anti-allergic effect of lactic acid bacteria isolated from seed mash used for brewing sake is not dependent on the total IgE levels. J Biosci Bioeng. 2012; 114(3):292–296. PMID: 22652086.
Article35. Kang H, Myung EJ, Ahn KS, Eom HJ, Han NS, Kim YB, Kim YJ, Sohn NW. Induction of Th1 cytokines by Leuconostoc mesenteroides subsp. mesenteroides (KCTC 3100) under Th2-type conditions and the requirement of NF-kappaB and p38/JNK. Cytokine. 2009; 46(2):283–289. PMID: 19299163.36. Kawakami T, Ando T, Kimura M, Wilson BS, Kawakami Y. Mast cells in atopic dermatitis. Curr Opin Immunol. 2009; 21(6):666–678. PMID: 19828304.
Article37. Galli SJ. Mast cells and basophils. Curr Opin Hematol. 2000; 7(1):32–39. PMID: 10608502.
Article38. Metz M, Piliponsky AM, Chen CC, Lammel V, Abrink M, Pejler G, Tsai M, Galli SJ. Mast cells can enhance resistance to snake and honeybee venoms. Science. 2006; 313(5786):526–530. PMID: 16873664.
Article39. McLachlan JB, Hart JP, Pizzo SV, Shelburne CP, Staats HF, Gunn MD, Abraham SN. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat Immunol. 2003; 4(12):1199–1205. PMID: 14595438.
Article40. Secor VH, Secor WE, Gutekunst CA, Brown MA. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J Exp Med. 2000; 191(5):813–822. PMID: 10704463.
Article41. Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007; 13(10):1211–1218. PMID: 17906636.
Article42. Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Snick JV, Strom TB, Zheng XX, Noelle RJ. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006; 442(7106):997–1002. PMID: 16921386.
Article43. Heissig B, Rafii S, Akiyama H, Ohki Y, Sato Y, Rafael T, Zhu Z, Hicklin DJ, Okumura K, Ogawa H, Werb Z, Hattori K. Low-dose irradiation promotes tissue revascularization through VEGF release from mast cells and MMP-9-mediated progenitor cell mobilization. J Exp Med. 2005; 202(6):739–750. PMID: 16157686.
Article44. Kim JE, Hwang IS, Goo JS, Nam SH, Choi SI, Lee HR, Lee YJ, Kim YH, Park SJ, Kim NS, Choi YH, Hwang DY. LP9M80-H isolated from Liriope platyphylla could help alleviate diabetic symptoms via the regulation of glucose and lipid concentration. J Life Sci. 2012; 22(5):634–641.45. Kim JE, Lee YK, Nam SH, Choi SI, Goo JS, Jang MJ, Lee HS, Son HJ, Lee CY, Hwang DY. The symptoms of atopic dermatitis in NC/Nga mice were significantly relieved by the water extract of Liriope platyphylla. Lab Anim Res. 2010; 26(4):377–384.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of IL-10 in the Trimellitic Anhydride-induced Contact Dermatitis
- A Case of Occupational Asthma Caused by Phthalic Anhydride
- The Symptoms of Atopic Dermatitis in NC/Nga Mice Were Significantly Relieved by the Water Extract of Liriope platyphylla
- Inhibitory Effect of Carnosol on Phthalic Anhydride-Induced Atopic Dermatitis via Inhibition of STAT3
- Combination Effect of Titrated Extract of Centella asiatica and Astaxanthin in a Mouse Model of Phthalic Anhydride-Induced Atopic Dermatitis