J Korean Med Sci.
2013 Feb;28(2):268-273. 10.3346/jkms.2013.28.2.268.
Lysophosphatidylcholine, Oxidized Low-Density Lipoprotein and Cardiovascular Disease in Korean Hemodialysis Patients: Analysis at 5 Years of Follow-up
- Affiliations
-
- 1Department of Internal Medicine, Hallym Kidney Research Institute, Hallym University College of Medicine, Seoul, Korea. jwn8671@unitel.co.kr
- 2Department of Laboratory Medicine, Hallym University College of Medicine, Seoul, Korea.
- 3Department of Pharmacology, Hallym University College of Medicine, Chuncheon, Korea.
- 4Division of Nephrology and Hypertension, Department of Medicine, University of California, Irvine, Irvine, California, USA.
- KMID: 1429196
- DOI: http://doi.org/10.3346/jkms.2013.28.2.268
Abstract
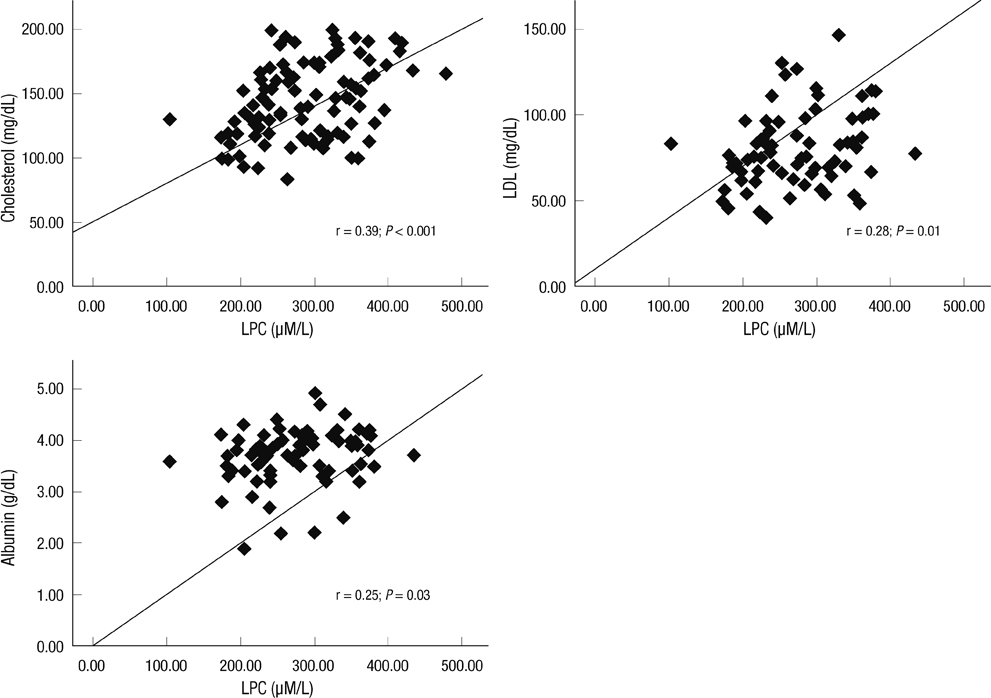
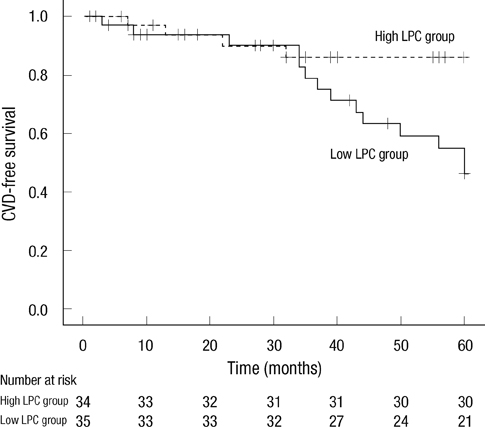
- Although oxidized low-density lipoprotein (LDL) and lysophosphatidylcholine (LPC) have been proposed as important mediators of the atherosclerosis, the long-term contribution to the risk of cardiovascular disease (CVD) in hemodialysis patients has not been evaluated. This study investigated the relation between oxidized LDL and LPC levels with long term risk of CVD. Plasma oxidized LDL and LPC levels were determined in 69 Korean hemodialysis patients as a prospective observational study for 5 yr. During the observation period, 18 cardiovascular events (26.1%) occurred including 6 deaths among the hemodialysis patients. The low LPC level group (< or = 254 microM/L, median value) had much more increased risk of CVD compared to the high LPC level group (> 254 microM/L) (P = 0.01). However, serum levels of oxidized LDL were not significantly different between groups with and without CVD. In adjusted Cox analysis, previous CVD, (hazard ratio [HR], 5.68; 95% confidence interval [CI], 1.94-16.63, P = 0.002) and low LPC level (HR, 3.45; 95% CI, 1.04-11.42, P = 0.04) were significant independent risk factors for development of CVD. It is suggested that low LPC, but not oxidized LDL, is associated with increased risk of CVD among a group of Korean hemodialysis patients.
Keyword
MeSH Terms
-
Adult
Aged
Asian Continental Ancestry Group
Cardiovascular Diseases/*diagnosis/etiology/mortality
Case-Control Studies
Female
Follow-Up Studies
Humans
Kidney Failure, Chronic/blood/complications/diagnosis
Lipoproteins, LDL/*blood
Lysophosphatidylcholines/*blood
Male
Middle Aged
Proportional Hazards Models
Prospective Studies
Renal Dialysis
Republic of Korea
Risk Factors
Lipoproteins, LDL
Lysophosphatidylcholines
Figure
Reference
-
1. Matsumoto T, Kobayashi T, Kamata K. Role of lysophosphatidylcholine (LPC) in atherosclerosis. Curr Med Chem. 2007. 14:3209–3220.2. Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989. 320:915–924.3. McIntyre TM, Zimmerman GA, Prescott SM. Biologically active oxidized phospholipids. J Biol Chem. 1999. 274:25189–25192.4. Vaziri ND. Dyslipidemia of chronic renal failure: the nature, mechanisms and potential consequences. Am J Physiol Renal Physiol. 2006. 290:F262–F272.5. Vaziri ND, Navab M, Fogelman AM. HDL metabolism and activity in chronic kidney disease. Nat Rev Nephrol. 2010. 6:287–296.6. Maggi E, Bellazzi R, Falaschi F, Frattoni A, Perani G, Finardi G, Gazo A, Nai M, Romanini D, Bellomo G. Enhanced LDL oxidation in uremic patients: an additional mechanism for accelerated atherosclerosis? Kidney Int. 1994. 45:876–883.7. Sasagawa T, Suzuki K, Shiota T, Kondo T, Okita M. The significance of plasma lysophospholipids in patients with renal failure on hemodialysis. J Nutr Sci Vitaminol. 1998. 44:809–818.8. Vuong TD, Stroes ES, Willekes-Koolschijn N, Rabelink TJ, Koomans HA, Joles JA. Hypoalbuminemia increases lysophosphatidylcholine in low-density lipoprotein of normocholesterolemic subjects. Kidney Int. 1999. 55:1005–1010.9. Kishimoto T, Soda Y, Matsuyama Y, Mizuno K. An enzymatic assay for lysophosphatidylcholine concentration in human serum and plasma. Clin Biochem. 2002. 35:411–416.10. Messner MC, Albert CJ, McHowat J, Ford DA. Identification of lysophosphatidylcholine-chlorohydrin in human atherosclerotic lesions. Lipids. 2008. 43:243–249.11. Lin P, Welch EJ, Gao XP, Malik AB, Ye RD. Lysophosphatidylcholine modulates neutrophil oxidant production through elevation of cyclic AMP. J Immunol. 2005. 174:2981–2989.12. Yan JJ, Jung JS, Lee JE, Lee J, Huh SO, Kim HS, Jung KC, Cho JY, Nam JS, Suh HW, et al. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat Med. 2004. 10:161–167.13. Drobnik W, Liebisch G, Audebert FX, Frohlich D, Gluck T, Vogel P, Rothe G, Schmitz G. Plasma ceramide and lysophosphatidylcholine inversely correlate with mortality in sepsis patients. J Lipid Res. 2003. 44:754–761.14. Schmitz G, Ruebsaamen K. Metabolism and atherogenic disease association of lysophosphatidylcholine. Atherosclerosis. 2010. 208:10–18.15. Thukkani AK, McHowat J, Hsu FF, Brennan ML, Hazen SL, Ford DA. Identification of alpha-chloro fatty aldehydes and unsaturated lysophosphatidylcholine molecular species in human atherosclerotic lesions. Circulation. 2003. 108:3128–3133.16. Jia L, Chen J, Yin P, Lu X, Xu G. Serum metabonomics study of chronic renal failure by ultra performance liquid chromatography coupled with Q-TOF mass spectrometry. Metabolomics. 2008. 4:183–189.17. Gillett MP, Obineche EN, Lakhani MS, Abdulle AM, Amirlak I, Al Rukhaimi M, Suleiman MN. Levels of cholesteryl esters and other lipids in the plasma of patients with end-stage renal failure. Ann Saudi Med. 2001. 21:283–286.18. Iseki K, Yamazato M, Tozawa M, Takishita S. Hypocholesterolemia is a significant predictor of death in a cohort of chronic hemodialysis patients. Kidney Int. 2002. 61:1887–1893.19. Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003. 63:793–808.20. Tsimikas S, Brilakis ES, Miller ER, McConnell JP, Lennon RJ, Kornman KS, Witztum JL, Berger PB. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med. 2005. 353:46–57.21. Bayés B, Pastor MC, Bonal J, Foraster A, Romero R. Oxidative stress, inflammation and cardiovascular mortality in haemodialysis--role of seniority and intravenous ferrotherapy: analysis at 4 years of follow-up. Nephrol Dial Transplant. 2006. 21:984–990.22. Kraśniak A, Drozdz M, Pasowicz M, Chmiel G, Michałek M, Szumilak D, Podolec P, Klimeczek P, Konieczyłska M, Wicher-Muniak E, et al. Factors involved in vascular calcification and atherosclerosis in maintenance haemodialysis patients. Nephrol Dial Transplant. 2007. 22:515–521.23. Nissel R, Faraj S, Sommer K, Henning L, van der Giet M, Querfeld U. Oxidative stress markers in young hemodialysis patients: a pilot study. Clin Nephrol. 2008. 70:135–143.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Associations between Oxidized LDL to LDL Ratio, HDL and Vascular Calcification in the Feet of Hemodialysis Patients
- Antibody Against Oxidized Low-Density Lipoprotein in Non-Insulin Dependent Diabetes Mellitus
- Increase of Intracellular Ca2+ Concentration Induced by Lysophosphatidylcholine in Murine Aortic Endothelial Cells
- Soluble Lectin-Like Oxidized Low-Density Lipoprotein Receptor 1 Is Inversely Correlated with the Activity of ANCA-Associated Vasculitis
- Oxidized Low-density Lipoprotein- and Lysophosphatidylcholine-induced Ca2+ Mobilization in Human Endothelial Cells