Korean J Radiol.
2004 Dec;5(4):240-249. 10.3348/kjr.2004.5.4.240.
Radiofrequency Ablation of Rabbit Liver In Vivo: Effect of the Pringle Maneuver on Pathologic Changes in Liver Surrounding the Ablation Zone
- Affiliations
-
- 1Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Korea. hklim@smc.samsung.co.kr
- 2Department of Pathology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Korea.
- 3Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Korea.
- 4Laboratory Animal Research Center, Samsung Biomedical Research Institute, Korea.
- KMID: 1118831
- DOI: http://doi.org/10.3348/kjr.2004.5.4.240
Abstract
OBJECTIVE
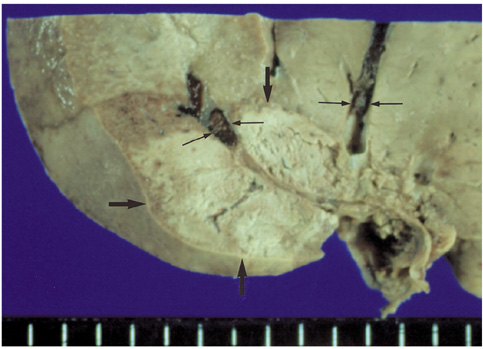
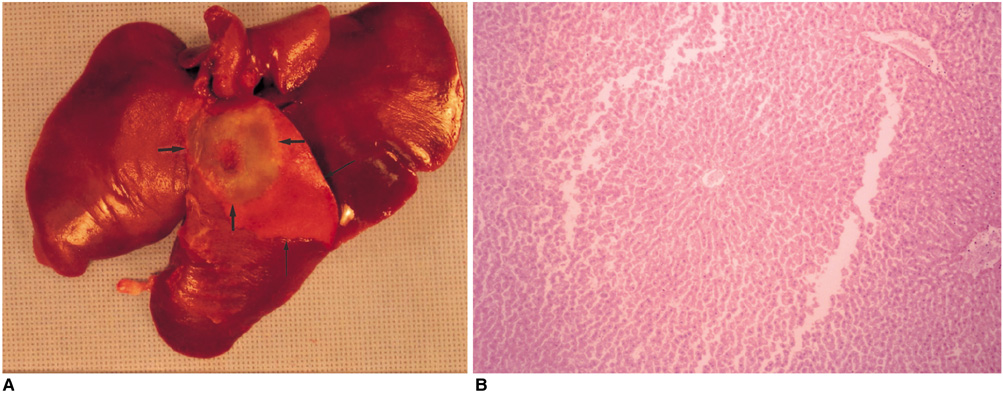
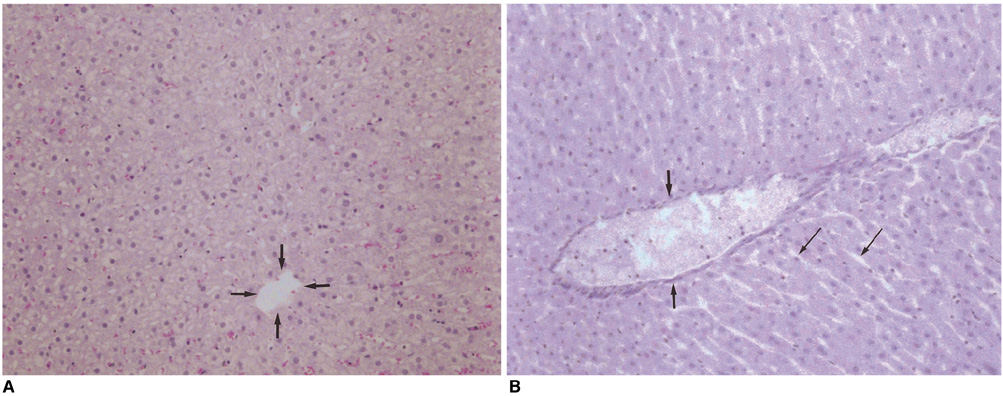
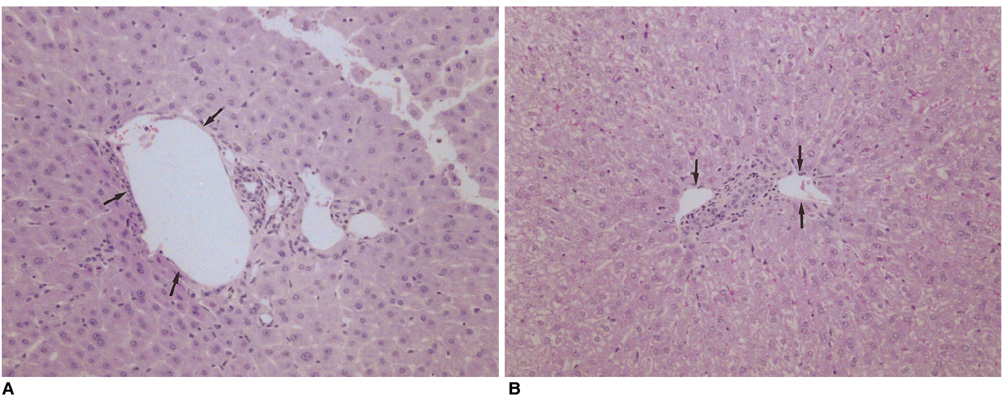
We wished to evaluate the effect of the Pringle maneuver (occlusion of both the hepatic artery and portal vein) on the pathologic changes in the hepatic vessels, bile ducts and liver parenchyma surrounding the ablation zone in rabbit livers. MATERIALS AND METHODS: Radiofrequency (RF) ablation zones were created in the livers of 24 rabbits in vivo by using a 50-W, 480-kHz monopolar RF generator and a 15-gauge expandable electrode with four sharp prongs for 7 mins. The tips of the electrodes were placed in the liver parenchyma near the porta hepatis with the distal 1 cm of their prongs deployed. Radiofrequency ablation was performed in the groups with (n=12 rabbits) and without (n=12 rabbits) the Pringle maneuver. Three animals of each group were sacrificed immediately, three days (the acute phase), seven days (the early subacute phase) and two weeks (the late subacute phase) after RF ablation. The ablation zones were excised and serial pathologic changes in the hepatic vessels, bile ducts and liver parenchyma surrounding the ablation zone were evaluated. RESULTS: With the Pringle maneuver, portal vein thrombosis was found in three cases (in the immediate [n=2] and acute phase [n=1]), bile duct dilatation adjacent to the ablation zone was found in one case (in the late subacute phase [n=1]), infarction adjacent to the ablation zone was found in three cases (in the early subacute [n=2] and late subacute [n=1] phases). None of the above changes was found in the livers ablated without the Pringle maneuver. On the microscopic findings, centrilobular congestion, sinusoidal congestion, sinusoidal platelet and neutrophilic adhesion, and hepatocyte vacuolar and ballooning changes in liver ablated with Pringle maneuver showed more significant changes than in those livers ablated without the Pringle maneuver (p < 0.05) CONCLUSION: Radiofrequency ablation with the Pringle maneuver created more severe pathologic changes in the portal vein, bile ducts and liver parenchyma surrounding the ablation zone compared with RF ablation without the Pringle maneuver. Therefore, we suggest that RF ablation with the Pringle maneuver should be performed with great caution in order to avoid unwanted thermal injury.
MeSH Terms
Figure
Reference
-
1. McGhana JP, Brock JM, Tesluk H, Gu WZ, Schneider P, Browning PD. Hepatic ablation with use of radio-frequency electrocautery in the animal model. J Vasc Interv Radiol. 1992. 3:291–297.2. Rossi S, Di Stasi M, Buscarini E, et al. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol. 1996. 167:759–768.3. Solbiati L, Ierace T, Goldberg SN, et al. Percutaneous US-guided radio-frequency tissue ablation of liver metastases: treatment and follow-up in 16 patients. Radiology. 1997. 202:195–203.4. Livraghi T, Goldberg SN, Monti F, et al. Saline-enhanced radiofrequency tissue ablation in the treatment of liver metastases. Radiology. 1997. 202:205–210.5. Solbiati L, Goldberg SN, Ierace T, et al. Hepatic metastases: percutaneous radio-frequency ablation with cooled-tip electrodes. Radiology. 1997. 205:367–373.6. Goldberg SN, Gazelle GS, Solbiati L, et al. Ablation of liver tumors using percutaneous RF therapy. AJR Am J Roentgenol. 1998. 170:1023–1028.7. Goldberg SN, Walovitch RC, Straub JA, Shore MT, Gazelle GS. Radio-frequency-induced coagulation necrosis in rabbits: immediate detection at US with a synthetic microsphere contrast agent. Radiology. 1999. 213:438–444.8. Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999. 230:1–8.9. Livraghi T, Goldberg SN, Lazzaroni S, et al. Hepatocellular carcinoma: radiofrequency ablation of medium and large lesions. Radiology. 2000. 214:761–768.10. Rossi S, Buscarini E, Garbagnati F, et al. Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. AJR Am J Roentgenol. 1998. 170:1015–1022.11. Sironi S, Livraghi T, Meloni F, De Cobelli F, Ferrero C, Del Maschio A. Small hepatocellular carcinoma treated with percutaneous RF ablation: MR imaging follow-up. AJR Am J Roentgenol. 1999. 173:1225–1229.12. Solbiati L, Goldberg SN, Ierace T, Dellanoce M, Livraghi T, Gazelle GS. Radio-frequency ablation of hepatic metastases: postprocedural assessment with a US microbubble contrast agent-early experience. Radiology. 1999. 211:643–649.13. Lim HK, Choi D, Lee WJ, et al. Hepatocellular carcinoma treated with radiofrequency ablation: evaluation with longterm follow-up multiphase helical CT. Radiology. 2001. 221:447–454.14. McGhana JP, Dodd GD 3rd. Radiofrequency ablation of the liver: current status. AJR Am J Roentgenol. 2001. 176:3–16.15. Lim HK. Radiofrequency thermal ablation of hepatocellular carcinomas. Korean J Radiol. 2000. 1:175–184.16. Dodd GD 3rd, Soulen MC, Kane RA, et al. Minimally invasive treatment of malignant hepatic tumors: at the threshold of a major breakthrough. RadioGraphics. 2000. 20:9–27.17. Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol. 2000. 174:323–331.18. Rhim H, Goldberg SN, Dodd GD 3rd, et al. Essential techniques for successful radio-frequency thermal ablation of malignant hepatic tumors. RadioGraphics. 2001. 21:S17–S35.19. Gilliams AR, Lees WR. The importance of large vessel proximity in thermal ablation of liver tumors (abstr). Radiology. 1999. 213(P):123.20. Patterson EJ, Scudamore CH, Owen DA, Nagy AG, Buczkowski AK. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg. 1998. 227:559–565.21. Rossi S, Garbagnati F, De Francesco I, et al. Relationship between the shape and size of radiofrequency induced thermal lesions and hepatic vascularization. Tumori. 1999. 85:128–132.22. Chinn SB, Lee FT Jr, Kennedy GD, et al. Effect of vascular occlusion on radiofrequency ablation of the liver: results in a porcine model. AJR Am J Roentgenol. 2001. 176:789–795.23. Chang CK, Hendy MP, Smith JM, Recht MH, Welling RE. Radiofrequency ablation of the porcine liver with complete hepatic vascular occlusion. Ann Surg Oncol. 2002. 9:594–598.24. Scott DJ, Fleming JB, Watumull LM, Lindberg G, Tesfay ST, Jones DB. The effect of hepatic inflow occlusion on laparoscopic radiofrequency ablation using simulated tumors. Surg Endosc. 2002. 16:1286–1291.25. Denys AL, De Baere T, Mahe C, et al. Radio-frequency tissue ablation of the liver: effects of vascular occlusion on lesion diameter and biliary and portal damages in a pig model. Eur Radiol. 2001. 11:2102–2108.26. Aschoff AJ, Merkle EM, Wong V, et al. How does alteration of hepatic blood flow affect liver perfusion and radiofrequency-induced thermal lesion size in rabbit liver? J Magn Reson Imaging. 2001. 13:57–63.27. Marchal F, Elias D, Rauch P, Leroux A, et al. Biliary lesions during radiofrequency ablation in liver. Study on the pig. Eur Surg Res. 2004. 36:88–94.28. Raman SS, Lu DS, Vodopich DJ, Sayre J, Lassman C. Creation of radiofrequency lesions in a porcine model: correlation with sonography, CT, and histopathology. AJR Am J Roentgenol. 2000. 175:1253–1258.29. Townsend CM, Beauchamp DR, Evers BM, Mattox KL. Hoyt DB, Coimbra R, Winchell RJ, editors. Sabiston textbook of surgery: the biological basis of modern surgical practice. Management of acute trauma. 2001. Philadelphia: WB Saunders;337–338.30. Rossi S, Garbagnati F, Lencioni R, et al. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000. 217:119–126.31. Man K, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg. 1997. 226:704–711.32. Man K, Fan S, Ng IO, et al. Tolerance of the liver to intermittent Pringle maneuver in hepatectomy for liver tumors. Arch Surg. 1999. 134:533–539.33. Jiao LR, Hansen PD, Havlik R, Mitry RR, Pignatelli M, Habib N. Clinical short-term results of radiofrequency ablation in primary and secondary liver tumors. Am J Surg. 1999. 177:303–306.34. Scudamore CH, Lee SI, Patterson EJ, et al. Radiofrequency ablation followed by resection of malignant liver tumors. Am J Surg. 1999. 177:411–417.35. Crawford JM. Kumar V, Abbas AK, Fausto N, editors. Liver and biliary tract. Robbins and Cotran pathologic basis of disease. 2004. 7th ed. Philadelphia: Elsevier Saunders;921.36. Crawford JM. Kumar V, Abbas AK, Fausto N, editors. Liver and biliary tract. Robbins and Cotran pathologic basis of disease. 2004. 7th ed. Philadelphia: Elsevier Saunders;880–881.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Internally Cooled Wet Electrode and Hepatic Vascular Inflow Occlusion Method for Hepatic Radiofrequency Ablation
- Radiofrequency Ablation with Epinephrine Injection: In Vivo Study in Normal Pig Livers
- Bipolar Radiofrequency Ablation Using Dual Internally Cooled Wet Electrodes: Experimental Study in Ex Vivo Bovine Liver
- Radiofrequency Ablation of Hepatic Cysts: Case Report
- Radiofrequency Thermal Ablation of Hepatocellular Carcinomas