Intravitreal Bevacizumab for Treatment of Diabetic Macular Edema
- Affiliations
-
- 1Department of Ophthalmology, Hallym University College of Medicine, Hallym University Sacred Heart Hospital, Anyang, Korea. piw@korea.com
- KMID: 1115646
- DOI: http://doi.org/10.3341/kjo.2009.23.1.17
Abstract
-
PURPOSE: To evaluate the effect of intravitreal bevacizumab on visual function and retinal thickness in patients with diabetic macular edema (DME).
METHODS
Thirty eyes of twenty-eight patients (mean age, 57.9+/-13.8 years) with DME were included in this study. Complete ophthalmic examination, including determination of best-corrected visual acuity (BCVA), stereoscopic biomicroscopy, and retinal thickness measurement by optical coherence tomography (OCT), was done at baseline and at each follow-up visit. All patients were treated with a 0.05 mL intravitreal injection containing 1.25 mg of bevacizumab.
RESULTS
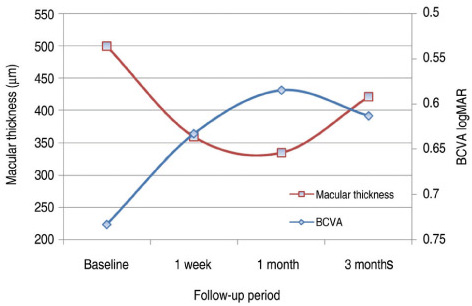
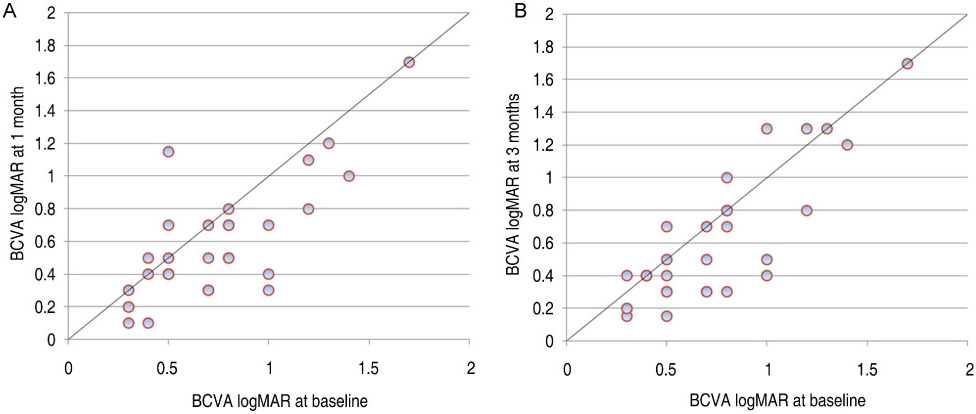
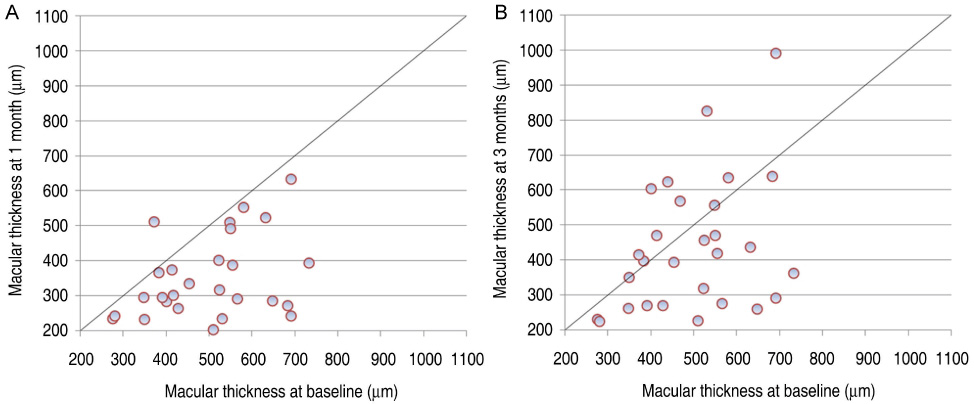
All patients completed 3 months of follow-up with a mean follow-up period of 5.26+/-2.39 months. The mean BCVA at baseline was 0.73+/-0.36 logMAR, which significantly improved to 0.63+/-0.41 (p=0.02), 0.58+/-0.36 (p=0.003), and 0.61+/-0.40 logMAR (p=0.006) at 1 week, 1 month, and 3 months. Final BCVA analysis demonstrated that 15 eyes (50%) remained stable and 12 (40%) improved > or =2 lines on BCVA. The mean central retinal thickness was 498.96+/-123.99 microm at baseline and decreased to 359.06+/-105.97 (p<0.001), 334.40+/-121.76 (p<0.001), 421.40+/-192.76 microm (p=0.035) at 1 week, 1 month, and 3 months. No ocular toxicity or adverse effects were observed.
CONCLUSIONS
Intravitreal bevacizumab injection resulted in significant improvement in BCVA and central retinal thickness as early as 1 week after injection in patients with DME, and this beneficial effect persisted for up to 3 months. However, the slight reduction in this improvement at 3 months suggests that repeated bevacizumab injections might be necessary. To evaluate the long-term safety and efficacy, further prospective randomized controlled clinical trials will be needed.
MeSH Terms
-
Angiogenesis Inhibitors/*administration & dosage
Antibodies, Monoclonal/*administration & dosage
Diabetic Retinopathy/*drug therapy/pathology
Female
Follow-Up Studies
Humans
Injections
Laser Therapy/methods
Macular Edema/*drug therapy/pathology
Male
Middle Aged
Retrospective Studies
Time Factors
Tomography, Optical Coherence
Treatment Outcome
Vascular Endothelial Growth Factor A/antagonists & inhibitors
Visual Acuity
Vitrectomy/methods
Vitreous Body
Figure
Cited by 4 articles
-
Combined Therapy of Intravitreal Bevacizumab and Posterior Subtenon Triamcinolone Acetonide Injection in Diabetic Macular Edema
Hoon Dong Kim, Kyung Seek Choi, Sung Jin Lee
J Korean Ophthalmol Soc. 2009;50(11):1652-1656. doi: 10.3341/jkos.2009.50.11.1652.Electrophysiological and Morphological Changes After Intravitreal Bevacizumab Injection with Macular Edema or Choroidal Neovascularization
Hyun Joon Lee, Joo Youn Park, Young-Hoon Ohn
J Korean Ophthalmol Soc. 2009;50(12):1824-1830. doi: 10.3341/jkos.2009.50.12.1824.Effects of Diabetic Retinopathy and Intravitreal Bevacizumab Injection on Choroidal Thickness in Diabetic Patients
Byeong Jun Park, Hye Won Chung, Hyung Chan Kim
J Korean Ophthalmol Soc. 2013;54(10):1520-1525. doi: 10.3341/jkos.2013.54.10.1520.The Results of a Combination of Cataract Surgery and Intravitreal Bevacizumab Injection for Diabetic Macular Edema
Bu Ki Kim, Eui Yong Kweon, Dong Wook Lee, Min Ahn, Nam Chun Cho
J Korean Ophthalmol Soc. 2010;51(7):954-960. doi: 10.3341/jkos.2010.51.7.954.
Reference
-
1. Ferris FL 3rd, Patz A. Macular edema: a complication of diabetic retinopathy. Surv Ophthalmol. 1984. 28:452–461.2. Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study Report Number 1. Arch Ophthalmol. 1985. 103:1796–1806.3. Jonas JB, Kreissig I, Sofker A, Degenring RF. Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol. 2003. 121:57–61.4. Martidis A, Duker JS, Greenberg PB, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002. 109:920–927.5. Jonas JB, Kreissig I, Degenring RF. Intraocular pressure after intravitreal injection of triamcinolone acetonide. Br J Ophthalmol. 2003. 87:24–27.6. Patelli F, Fasolino G, Radice P, et al. Time course of changes in retinal thickness and visual acuity after intravitreal triamcinolone acetonide for diffuse diabetic macular edema with and without previous macular laser treatment. Retina. 2005. 26:840–845.7. Qaum T, Xu Q, Joussen AM, et al. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci. 2001. 42:2408–2413.8. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994. 331:1480–1487.9. Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994. 118:445–450.10. Funatsu H, Yamashita H, Noma H, et al. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol. 2002. 133:70–77.11. Funatsu H, Yamashita H, Ikeda T, et al. Angiotensin II and vascular endothelial growth factor in the vitreous fluid of patients with diabetic macular edema and other retinal disorders. Am J Ophthalmol. 2002. 133:537–543.12. Cunningham ET Jr, Adamis AP, Altaweel M, et al. A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology. 2005. 112:1747–1757.13. Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004. 3:391–400.14. Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006. 26:275–278.15. Michels S, Rosenfeld PJ, Puliafito CA, et al. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration. Twelve-week results of an uncontrolled open-label clinical study. Ophthalmology. 2005. 112:1035–1047.16. Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006. 113:363–372.17. Iturralde D, Spaide RF, Meyerle CB, et al. Intravitreal bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: a short-term study. Retina. 2006. 26:279–284.18. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulindependent diabetes mellitus. N Engl J Med. 1993. 329:977–986.19. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998. 317:703–713.20. Strom C, Sander B, Klemp K, et al. Effect of ruboxistaurin on blood-retinal barrier permeability in relation to severity of leakage in diabetic macular edema. Invest Ophthalmol Vis Sci. 2005. 46:3855–3858.21. Campochiaro PA. C99-PKC412-003 Study Group. Reduction of diabetic macular edema by oral administration of the kinase inhibitor PKC412. Invest Ophthalmol Vis Sci. 2004. 45:922–931.22. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004. 25:581–611.23. Mordenti J, Thomsen K, Licko V, et al. Intraocular pharmacokinetics and safety of a humanized monoclonal antibody in rabbits after intravitreal administration of a solution or a PLGA microsphere formulation. Toxicol Sci. 1999. 52:101–106.24. Shahar JS, Avry RL, Heilweil G, et al. Electrophysiologic and retinal penetration studies following intravitreal injection of bevacizumab. Retina. 2006. 26:262–269.25. Maturi RK, Bleau LA, Wilson DL. Electrophysiologic findings after intravitreal bevacizumab (Avastin) treatment. Retina. 2006. 26:270–274.26. Manzano RP, Peyman GA, Khan P, Kivilcim M. Testing intravitreal toxicity of bevacizumab (Avastin). Retina. 2006. 26:257–261.27. Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005. 36:331–335.28. Haritoglou C, Kook D, Neubauer A, et al. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006. 26:999–1005.29. Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, et al. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema. Ophthalmology. 2007. 114:743–750.30. Yanyali A, Aytug B, Horozoglu F, Nohutcu AF. Bevacizumab (Avastin) for diabetic macular edema in previously vitrectomized eyes. Am J Ophthalmol. 2007. 144:124–126.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Macular Hole Formation after Intravitreal Injection of Bevacizumab for Diabetic Macular Edema
- Comparison of Intravitreal Bevacizumab Alone Injection and Intravitreal Combination Low-Dose Bevacizumab-Triamcinolone Injection or Diabetic Macular Edema
- A Multimodal Approach to Diabetic Macular Edema
- The Effects of Intravitreal Bevacizumab Injection According to the Type of Diabetic Macular Edema
- Short-Term Results of Dexamethasone Intravitreal Implant in Patients with Refractory Diabetic Macular Edema