J Korean Ophthalmol Soc.
2014 Aug;55(8):1155-1161.
Comparison of Intravitreal Bevacizumab Alone Injection and Intravitreal Combination Low-Dose Bevacizumab-Triamcinolone Injection or Diabetic Macular Edema
- Affiliations
-
- 1Department of Ophthalmology, Gyeongsang National University School of Medicine, Jinju, Korea. medcabin@hanmail.net
- 2Gyeongsang Institute of Health Science, Gyeongsang National University, Jinju, Korea.
Abstract
- PURPOSE
To report the effects and intraocular pressure results of intravitreal bevacizumab alone injection compared with intravitreal low-dose bevacizumab combined with low-dose triamcinolone injection in patients with diabetic macular edema.
METHODS
In total, 40 eyes of 40 patients diagnosed with diabetic macular edema were evaluated. Of these, 20 eyes of 20 patients were injected with intravitreal bevacizumab (1.25 mg/0.05 mL) and 20 eyes of 20 patients were injected with low-dose bevacizumab (0.625 mg/0.025 mL) combined with low-dose triamcinolone (1 mg/0.025 mL). The best corrected visual acuity (BCVA), central macular thickness, and intraocular pressure of treated eyes were measured before injection and at 1 month, 2 months, and 3 months after injection.
RESULTS
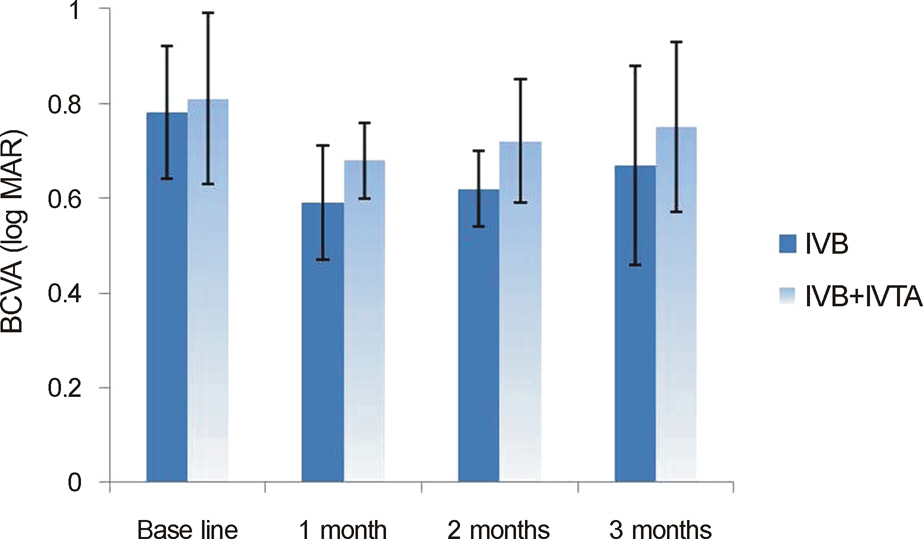
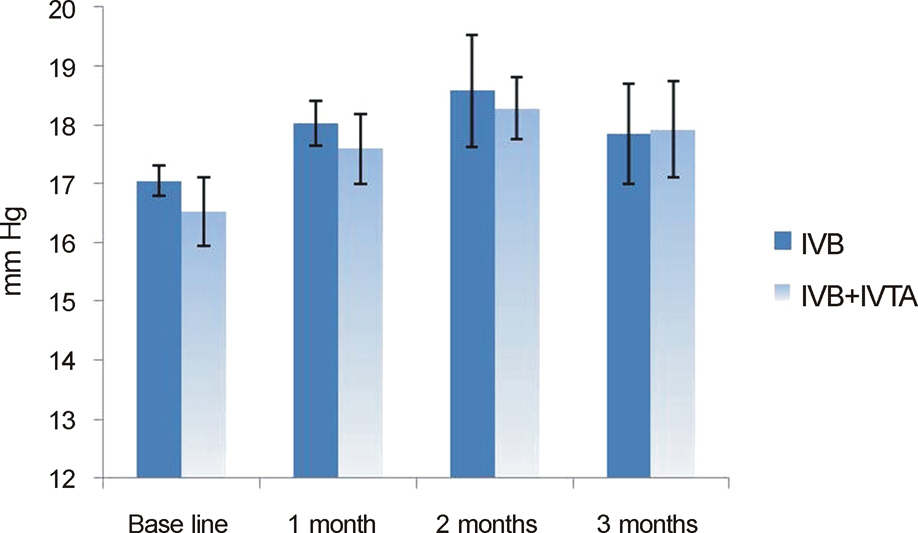
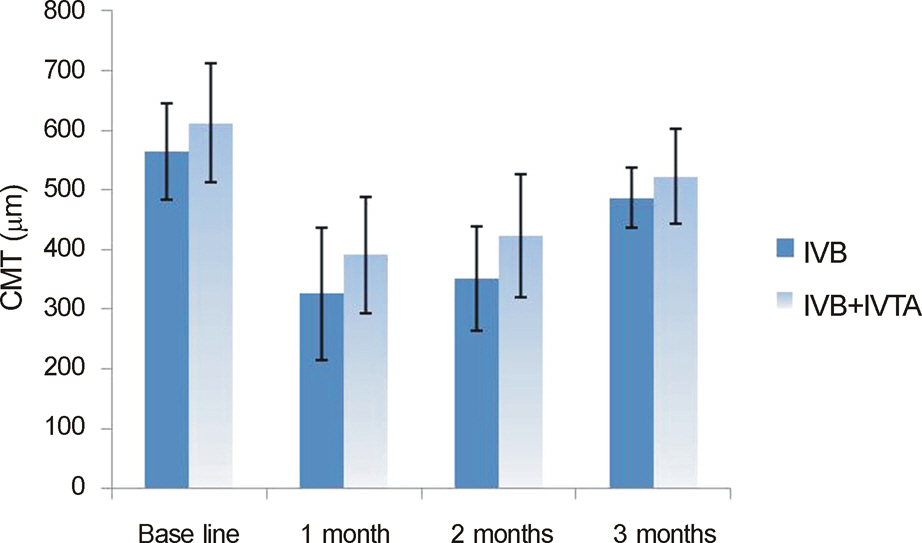
In both the intravitreal bevacizumab and the low-dose bevacizumab combined with low-dose triamcinolone groups, BCVA increased significantly at 1 month, 2 months, and 3 months after injection (p < 0.05). In addition, in both groups, neither intraocular pressure (IOP) nor central macular thickness increased significantly at 1 month, 2 months, or 3 months after injection (p > 0.05). The BCVA, IOP, and central macular thickness (CMT) at 1 month, 2 months, and 3 months after injection showed no significant differences between the intravitreal bevacizumab group and the low-dose bevacizumab combined with low-dose triamcinolone group (p > 0.05).
CONCLUSIONS
The BCVA of both groups increased significantly, and the CMT of both groups decreased significantly in patients with diabetic macular edema. The injection of low-dose intravitreal bevacizumab combined with low-dose intravitreal triamcinolone may be useful for the treatment of diabetic macular edema.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Paccola L, Costa RA, Folgosa MS, et al. Intravitreal triamcinolone versus bevacizumab for treatment of refractory diabetic macular oedema (IBEME study). Br J Ophthalmol. 2008; 92:76–80.
Article2. Massin P, Audren F, Haouchine B, et al. Intravitreal triamcinolone acetonide for diabeitc macular edema: preliminary results of a prospective controlled trail. Ophthalmology. 2004; 111:218–24. discussion 224-5.3. Fischer S, Renz D, Schaper W, Karliczek GF. In vitro effects of dexamethasone on hypoxia-induced hyperpermeability and expression of vascular endothelial growth factor. Eur J Phamacol. 2001; 411:231–43.
Article4. McCuen BW 2nd, Bessler M, Tano Y, et al. The lack of toxicity of intravitreally administered triamcinolone acetonide. Am J Ophthalmol. 1981; 91:785–8.
Article5. Moshfeghi DM, Kaiser PK, Scott IU, et al. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2003; 136:791–6.
Article6. Nelson ML, Tennant MT, Sivalingam A, et al. Infectious and presumed noninfectious endophthalmitis after intravitreal triamcinolone acetonide injection. Retina. 2003; 23:686–91.
Article7. Roth DB, Chieh J, Spirn MJ, et al. Noninfectious endophthalmitis associated with intravitreal triamcinolone injection. Arch Ophthalmol. 2003; 121:1279–82.
Article8. Jonas JB, Spandau UH, Schlichtenbrede F. Short-term complications of intravitreal injections of triamcinolone and bevacizumab. Eye (Lond). 2008; 22:590–1.
Article9. Pieramici DJ, Avery RL, Castellarin AA, et al. Case of anterior uveitis after intravitreal injection of bevacizumab. Retina. 2006; 26:841–2.
Article10. Meyer CH, Mennel S, Schmidt JC, Kroll P. Acute retinal pigment epithelial tear following intravitreal bevacizumab (Avastin) injection for occult choroidal neovascularization seconday to age related macular degeneration. Br J Ophthalmol. 2006; 90:1207–8.11. Wang YS, LI X, Wang HY, et al. Intravitreal bevacizumab combined with/without triamcinolone acetonide in single injection for treatment of diabetic macular edema. Chin Med J (Engl). 2011; 124:352–8.12. Chang MW, Kim SW, Oh IK, et al. Intravitreal tamcinolone injection with or without bevacizumab for diabetic macular edema. J Korean Ophthalmol Soc. 2008; 49:1269–74.13. Gillies MC, Sutter FK, Simpson JM, et al. Intravitreal triamcinolone for refractory diabetic macular edema: two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology. 2006; 113:1533–8.14. Arevalo JF, Garcia-Amaris RA. Intravitreal bevacizumab for diabetic retinopathy. Curr Diabetes Rev. 2009; 5:39–46.
Article15. Hesse L, Grusser M, Hoffstadt K, et al. Population-based study of diabetic retinopathy in Wolfsburg. Ophthalmologe. 2001; 98:1065–8.16. Gaudric A, Massin-Korobelnik P. Diabetic maculopathy: classification, epidemiology, spontaneous outcome, treatment. Diabete Metab. 1993; 19:422–9.17. Agardh E, Hultberg B, Agardh C. Effects of inhibition of glycation and oxidative stress on the development of cataract and retinal vessel abnormalities in diabetic rats. Curr Eye Res. 2000; 21:543–9.
Article18. Ryan SJ, Hinton DR, Schachat AP, et al. Nonproliferative diabetic retinopathy. In : Chew EY, Ferris FL, editors. Retina. 4th ed.New York: Mosby;2006. v. 2. chap. 67.19. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331:1480–7.
Article20. Kim SJ. Diabetic macular edema. Ophthalmology. 2009; 116:1228.
Article21. Funatsu H, Noma H, Mimura T, et al. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology. 2009; 116:73–9.
Article22. Shimura M, Nakazawa T, Yasuda K, et al. Comparative therapy evaluation of intravitreal bevacizumab and triamcinolone acetonide on persistent diffuse diabetic macular edema. Am J Ophthalmol. 2008; 145:854–61.
Article23. Antcliff RJ, Marshall J. The pathogenesis of edema in diabetic maculopathy. Semin Ophthalmol. 1999; 14:223–32.
Article24. Audren F, Lecleire-Collet A, Erginay A, et al. Intravitreal triamcinolone acetonide for diffuse diabetic macular edema: phase 2 trial comparing 4 mg vs 2 mg. Am J Ophthalmol. 2006; 142:794–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intravitreal Triamcinolone Injection with or Without Bevacizumab for Diabetic Macular Edema
- Combined Low Dose Bevacizumab-triamcinolone versus Bevacizumab Single Intravitreal Injection for Branch Retinal Vein Occlusion
- Optical Coherence Tomography Angiography in Refractory Diabetic Macular Edema
- Intravitreal Triamcinolone Versus Bevacizumab for Treatment of Diabetic Macular Edema
- Effect of High-dose Intravitreal Bevacizumab Injection on Refractory Idiopathic Choroidal Neovasculariz