J Vet Sci.
2011 Jun;12(2):107-113. 10.4142/jvs.2011.12.2.107.
Real time observation of mouse fetal skeleton using a high resolution X-ray synchrotron
- Affiliations
-
- 1Department of Radiology, College of Veterinary Medicine, Chungbuk National University, Cheongju 361-763, Korea.
- 2Medical Research Center, College of Medicine, Yonsei University, Seoul 120-752, Korea.
- 3Animal Science Branch, National Cancer Center, Koyang 410-769, Korea.
- 4Department of Materials Sciences, Pohang University of Science and Technology, Pohang 790-784, Korea.
- 5Department of Anatomy and Cell Biology, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. snumouse@snu.ac.kr
- 6Department of Radiology, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea.
- 7BK21 Program for Veterinary Science, Seoul National University, Seoul 151-742, Korea.
- 8Research Institute for Veterinary Science, Seoul National University, Seoul 151-742, Korea.
- 9Department of Internal Medicine, College of Veterinary Medicine, Jeju National University, Jeju 690-756, Korea.
- 10Institute of Physics, Academia Sinica, 128 Academic Rd., Nankang, Taipei 11529, Taiwan.
- KMID: 1106551
- DOI: http://doi.org/10.4142/jvs.2011.12.2.107
Abstract
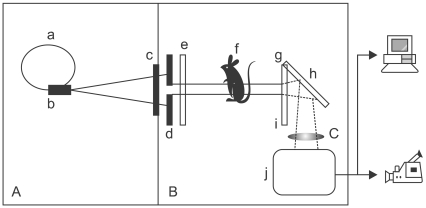
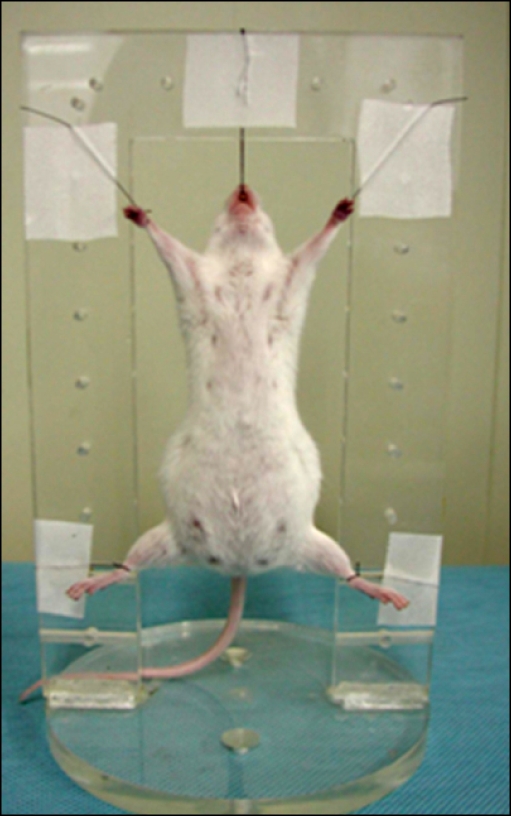
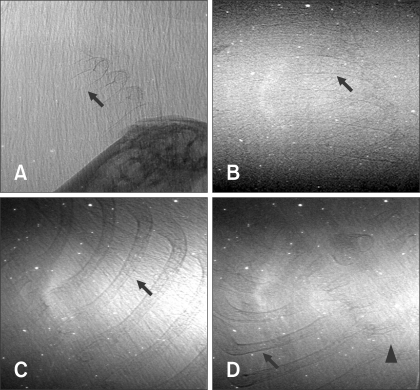
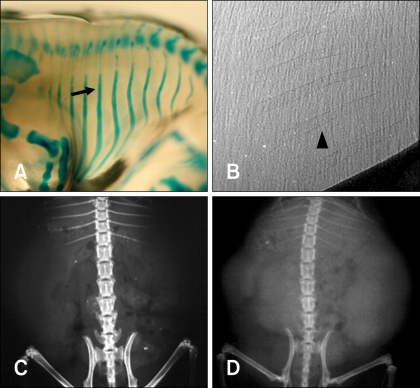
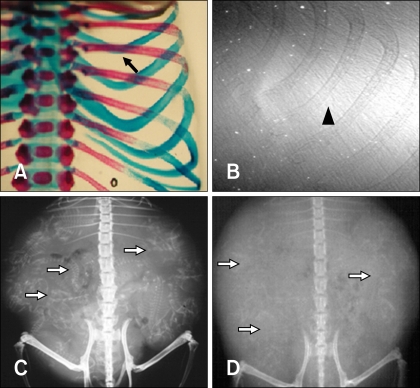
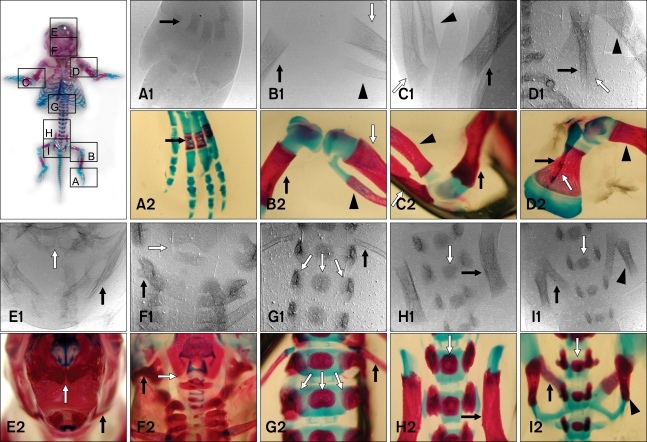
- The X-ray synchrotron is quite different from conventional radiation sources. This technique may expand the capabilities of conventional radiology and be applied in novel manners for special cases. To evaluate the usefulness of X-ray synchrotron radiation systems for real time observations, mouse fetal skeleton development was monitored with a high resolution X-ray synchrotron. A non-monochromatized X-ray synchrotron (white beam, 5C1 beamline) was employed to observe the skeleton of mice under anesthesia at embryonic day (E)12, E14, E15, and E18. At the same time, conventional radiography and mammography were used to compare with X-ray synchrotron. After synchrotron radiation, each mouse was sacrificed and stained with Alizarin red S and Alcian blue to observe bony structures. Synchrotron radiation enabled us to view the mouse fetal skeleton beginning at gestation. Synchrotron radiation systems facilitate real time observations of the fetal skeleton with greater accuracy and magnification compared to mammography and conventional radiography. Our results show that X-ray synchrotron systems can be used to observe the fine structures of internal organs at high magnification.
Keyword
MeSH Terms
Figure
Reference
-
1. Baatout S, Jacquet P, Michaux A, Buset J, Vankerkom J, Derradji H, Yan J, von Suchodoletz H, de Saint-Georges L, Desaintes C, Mergeay M. Developmental abnormalities induced by X-irradiation in p53 deficient mice. In Vivo. 2002; 16:215–221. PMID: 12182118.2. Bailey LJ, Johnston MC, Billet J. Effects of carbon monoxide and hypoxia on cleft lip in A/J mice. Cleft Palate Craniofac J. 1995; 32:14–19. PMID: 7727482.
Article3. Farley FA, Hall J, Goldstein SA. Characteristics of congenital scoliosis in a mouse model. J Pediatr Orthop. 2006; 26:341–346. PMID: 16670546.
Article4. Guldberg RE, Lin ASP, Coleman R, Robertson G, Duvall C. Microcomputed tomography imaging of skeletal development and growth. Birth Defects Res C Embryo Today. 2004; 72:250–259. PMID: 15495187.
Article5. Hwu Y, Hsieh HH, Lu MJ, Tsai WL, Lin HM, Goh WC, Lai B, Je JH, Kim CK, Noh DY. Coherence-enhanced synchrotron radiology: Refraction versus diffraction mechanisms. J Appl Phys. 1999; 86:4613–4618.
Article6. Hwu Y, Lai B, Mancini DC, Je JH, Noh DY, Bertolo M, Tromba G, Margaritondo G. Coherence based contrast enhancement in x-ray radiography with a photoelectron microscope. Appl Phys Lett. 1999; 75:2377–2379.
Article7. Hwu Y, Tsai WL, Lai B, Mancini DC, Je JH, Noh DY, Youn HS, Hwang CS, Cerrina F, Swiech W, Bertolo M, Tromba G, Margaritondo G. Use of photoelectron microscopes as X-ray detectors for imaging and other applications. Nucl Instrum Methods Phys Res A. 1999; 437:516–520.
Article8. Hwu Y, Tsai WL, Je JH, Seol SK, Kim B, Groso A, Margaritondo G, Lee KH, Seong JK. Synchrotron microangiography with no contrast agent. Phys Med Biol. 2004; 49:501–508. PMID: 15005160.
Article9. Itai Y, Takeda T. Application of synchrotron radiation to medicine. Two-dimensional intravenous coronary arteriography and monochromatic x-ray computed tomography. Invest Radiol. 1993; 28(Suppl 3):S158–S159. PMID: 8376044.10. Lee KH, Hwu YK, Je JH, Tsai WL, Choi EW, Kim YC, Kim HJ, Seong JK, Yi SW, Ryo HS, Margaritondo G. Synchrotron radiation imaging of internal structures in live animals. Yonsei Med J. 2002; 43:25–30. PMID: 11854928.
Article11. Lewis R. Medical applications of synchrotron radiation x-rays. Phys Med Biol. 1997; 42:1213–1243. PMID: 9253036.
Article12. Loder RT, Hernandez MJ, Lerner AL, Winebrener DJ, Goldstein SA, Hensinger RN, Liu CY, Schork MA. The induction of congenital spinal deformities in mice by maternal carbon monoxide exposure. J Pediatr Orthop. 2000; 20:662–666. PMID: 11008750.
Article13. Meuli R, Hwu Y, Je JH, Margaritondo G. Synchrotron radiation in radiology: radiology techniques based on synchrotron sources. Eur Radiol. 2004; 14:1550–1560. PMID: 15316744.
Article14. Momose A, Takeda T, Itai Y. Blood vessels: depiction at phase-contrast X-ray imaging without contrast agents in the mouse and rat-feasibility study. Radiology. 2000; 217:593–596. PMID: 11058666.
Article15. Mori H, Hyodo K, Tobita K, Chujo M, Shinozaki Y, Sugishita Y, Ando M. Visualization of penetrating transmural arteries in situ by monochromatic synchrotron radiation. Circulation. 1994; 89:863–871. PMID: 8313576.16. Murray FJ, Schwetz BA, Crawford AA, Henck JW, Quast JF, Staples RE. Embryotoxicity of inhaled sulfur dioxide and carbon monoxide in mice and rabbits. J Environ Sci Health C. 1979; 13:233–250. PMID: 555466.17. Neggers YH, Singh J. Zinc supplementation to protein-deficient diet in CO-exposed mice decreased fetal mortality and malformation. Biol Trace Elem Res. 2006; 114:269–279. PMID: 17206008.18. Oest ME, Jones JC, Hatfield C, Prater MR. Micro-CT evaluation of murine fetal skeletal development yields greater morphometric precision over traditional clear-staining methods. Birth Defects Res B Dev Reprod Toxicol. 2008; 83:582–589. PMID: 19048632.
Article19. Ohtsuka S, Sugishita Y, Takeda T, Itai Y, Hyodo K, Ando M. Dynamic intravenous coronary arteriography using synchrotron radiation and its application to the measurement of coronary blood flow. Jpn Circ J. 1997; 61:432–440. PMID: 9192243.
Article20. Ohyama K, Chung CH, Chen E, Gibson CW, Misof K, Fratzl P, Shapiro IM. p53 influences mice skeletal development. J Craniofac Genet Dev Biol. 1997; 17:161–171. PMID: 9493073.21. Schültke E, Fiedler S, Nemoz C, Ogieglo L, Kelly ME, Crawford P, Esteve F, Brochard T, Renier M, Requardt H, Le Duc G, Juurlink B, Meguro K. Synchrotron-based intra-venous K-edge digital subtraction angiography in a pig model: A feasibility study. Eur J Radiol. 2010; 73:677–681. PMID: 19233584.
Article22. Schwetz BA, Smith FA, Leong BKJ, Staples RE. Teratogenic potential of inhaled carbon monoxide in mice and rabbits. Teratology. 1979; 19:385–392. PMID: 473091.
Article23. Singh J, Aggison L Jr, Moore-Cheatum L. Teratogenicity and developmental toxicity of carbon monoxide in protein-deficient mice. Teratology. 1993; 48:149–159. PMID: 8211821.
Article24. Suortti P, Thomlinson W. Medical applications of synchrotron radiation. Phys Med Biol. 2003; 48:R1–R35. PMID: 12884920.
Article25. Tanaka E, Tanaka A, Sekka T, Shinozaki Y, Hyodo K, Umetani K, Mori H. Digitized cerebral synchrotron radiation angiography: quantitative evaluation of the canine circle of Willis and its large and small branches. AJNR Am J Neuroradiol. 1999; 20:801–806. PMID: 10369349.26. Wise LD, Winkelmann CT. Micro-computed tomography and alizarin red evaluations of boric acid-induced fetal skeletal changes in Sprague-Dawley rats. Birth Defects Res B Dev Reprod Toxicol. 2009; 86:214–219. PMID: 19479792.
Article27. Wise LD, Winkelmann CT. Evaluation of hydroxyurea-induced fetal skeletal changes in Dutch belted rabbits by micro-computed tomography and alizarin red staining. Birth Defects Res B Dev Reprod Toxicol. 2009; 86:220–226. PMID: 19479798.
Article28. Zimmerman EF, Scott WJ Jr, Collins MD. Ethanol-induced limb defects in mice: effect of strain and Ro15-4513. Teratology. 1990; 41:453–462. PMID: 2160130.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Synchrotron Radiation Imaging of Breast Tissue Using a Phase-contrast Hard X-ray Microscope
- Phase Contrast Microradiography of Mouse Lung Using Synchrotron X-ray: Correlation with Optical Microscopy
- Synchrotron X-ray Microscopic Imaging of Thyroid Tissues
- Synchrotron Radiation Imaging of Female Breast Tissues Using Phase Contrast Technique
- Optical Imaging Technology for Real-time Tumor Monitoring