Yonsei Med J.
2011 Jan;52(1):51-58. 10.3349/ymj.2011.52.1.51.
Epithelial to Mesenchymal Transition of Mesothelial Cells in Tuberculous Pleurisy
- Affiliations
-
- 1Department of Internal Medicine, Hallym University College of Medicine, Chuncheon, Korea. dongyu@hallym.ac.kr
- 2Department of Pathology, Hallym University College of Medicine, Chuncheon, Korea.
- 3Department of Thoracic & Cardiovascular Surgery, Hallym University College of Medicine, Chuncheon, Korea.
- 4Lung Research Institute of Hallym University College of Medicine, Chuncheon, Korea.
- KMID: 1106437
- DOI: http://doi.org/10.3349/ymj.2011.52.1.51
Abstract
- PURPOSE
Tuberculous pleurisy is the most frequent extrapulmonary manifestation of tuberculosis. In spite of adequate treatment, pleural fibrosis is a common complication, but the mechanism has not been elucidated. This study is to determine whether epithelial to mesenchymal transition (EMT) of mesothelial cells occurs in tuberculous pleurisy.
MATERIALS AND METHODS
Normal pleural mesothelial cells, isolated from irrigation fluids during operations for primary spontaneous pneumothorax, were characterized by immunofluorescence and reverse transcription polymerase chain reaction (RT-PCR). These cells were treated in vitro with various cytokines, which were produced in the effluents of tuberculous pleurisy. The isolated cells from the effluents of tuberculous pleurisy were analyzed by immunofluorescence and RT-PCR analysis.
RESULTS
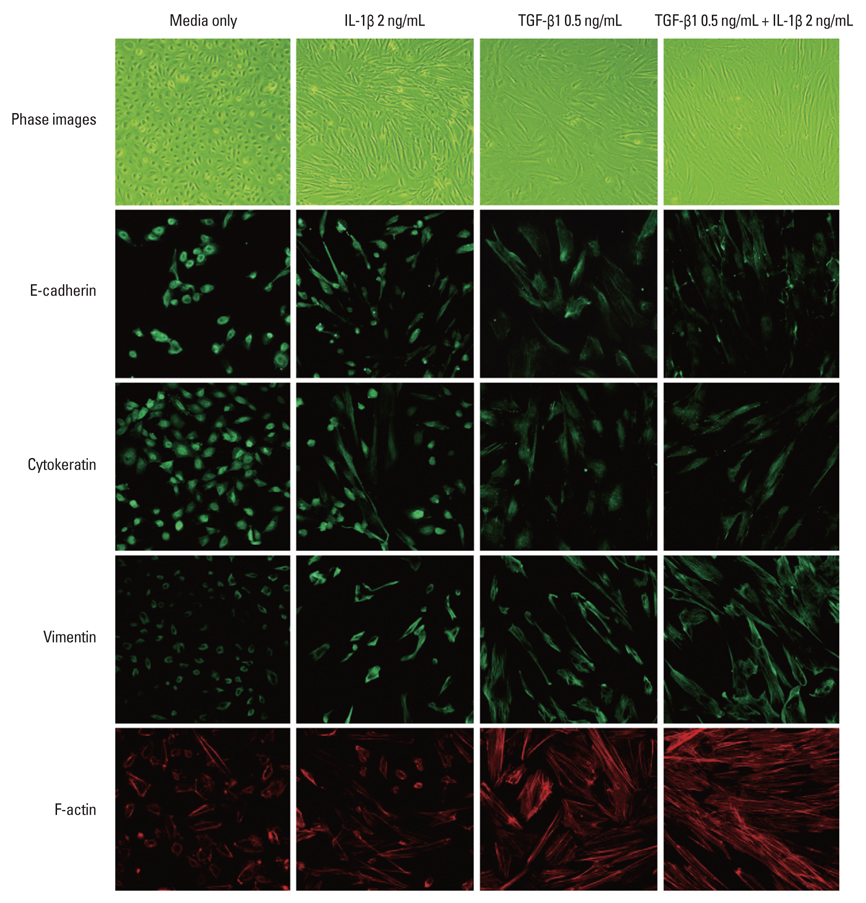
The isolated cells from the irrigation fluid of primary spontaneous pneumothorax had epithelial characteristics. These cells, with transforming growth factor-beta1 and/or interleukin-1beta treatment, underwent phenotypic transition from epithelial to mesenchymal cells, with the loss of epithelial morphology and reduction in cytokeratin and E-cadherin expression. Effluent analysis from tuberculous pleurisy using immunofluorescence and RT-PCR demonstrated two phenotypes that showed mesenchymal characteristics and both epithelial & mesencymal characteristics.
CONCLUSION
Our results suggest that pleural mesothelial cells in tuberculous pleurisy have been implicated in pleural fibrosis through EMT.
Keyword
MeSH Terms
Figure
Reference
-
1. Seibert AF, Haynes J Jr, Middleton R, Bass JB Jr. Tuberculous pleural effusion. Twenty-year experience. Chest. 1991. 99:883–886.2. de Pablo A, Villena V, Echave-Sustaeta J, Encuentra AL. Are pleural fluid parameters related to the development of residual pleural thickening in tuberculosis? Chest. 1997. 112:1293–1297.
Article3. Huggins JT, Sahn SA. Causes and management of pleural fibrosis. Respirology. 2004. 9:441–447.
Article4. Mutsaers SE, Prele CM, Brody AR, Idell S. Pathogenesis of pleural fibrosis. Respirology. 2004. 9:428–440.
Article5. Bariety J, Hill GS, Mandet C, Irinopoulou T, Jacquot C, Meyrier A, et al. Glomerular epithelial-mesenchymal transdifferentiation in pauci-immune crescentic glomerulonephritis. Nephrol Dial Transplant. 2003. 18:1777–1784.
Article6. Nicolás FJ, Lehmann K, Warne PH, Hill CS, Downward J. Epithelial to mesenchymal transition in Madin-Darby canine kidney cells is accompanied by down-regulation of Smad3 expression, leading to resistance to transforming growth factor-beta-induced growth arrest. J Biol Chem. 2003. 278:3251–3256.
Article7. Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol. 2001. 159:1465–1475.
Article8. Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002. 110:341–350.
Article9. Strutz F, Zeisberg M, Ziyadeh FN, Yang CQ, Kalluri R, Müller GA, et al. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int. 2002. 61:1714–1728.
Article10. Yáñez-Mó M, Lara-Pezzi E, Selgas R, Ramírez-Huesca M, Domínguez-Jiménez C, Jiménez-Heffernan JA, et al. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N Engl J Med. 2003. 348:403–413.
Article11. Selgas R, Bajo A, Jiménez-Heffernan JA, Sánchez-Tomero JA, Del Peso G, Aguilera A, et al. Epithelial-to-mesenchymal transition of the mesothelial cell--its role in the response of the peritoneum to dialysis. Nephrol Dial Transplant. 2006. 21:Suppl 2. ii2–ii7.
Article12. Aguilera A, Yáñez-Mó M, Selgas R, Sánchez-Madrid F, López-Cabrera M. Epithelial to mesenchymal transition as a triggering factor of peritoneal membrane fibrosis and angiogenesis in peritoneal dialysis patients. Curr Opin Investig Drugs. 2005. 6:262–268.13. Maeda J, Ueki N, Ohkawa T, Iwahashi N, Nakano T, Hada T, et al. Local production and localization of transforming growth factor-beta in tuberculous pleurisy. Clin Exp Immunol. 1993. 92:32–38.
Article14. Yanagawa H, Yano S, Haku T, Ohmoto Y, Sone S. Interleukin-1 receptor antagonist in pleural effusion due to inflammatory and malignant lung disease. Eur Respir J. 1996. 9:1211–1216.
Article15. Kroegel C, Antony VB. Immunobiology of pleural inflammation: potential implications for pathogenesis, diagnosis and therapy. Eur Respir J. 1997. 10:2411–2418.
Article16. Kunz CR, Jadus MR, Kukes GD, Kramer F, Nguyen VN, Sasse SA. Intrapleural injection of transforming growth factor-beta antibody inhibits pleural fibrosis in empyema. Chest. 2004. 126:1636–1644.
Article17. Ceyhan BB, Demiralp E, Karakurt ZL, Karakurt S, Sungur M. Transforming growth factor beta-1 level in pleural effusion. Respirology. 2003. 8:321–325.
Article18. Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003. 15:740–746.
Article19. Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel). 1995. 154:8–20.
Article20. Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003. 9:964–968.
Article21. Khalil N, Parekh TV, O'Connor R, Antman N, Kepron W, Yehaulaeshet T, et al. Regulation of the effects of TGF-beta 1 by activation of latent TGF-beta 1 and differential expression of TGF-beta receptors (T beta R-I and T beta R-II) in idiopathic pulmonary fibrosis. Thorax. 2001. 56:907–915.
Article22. Takizawa H, Tanaka M, Takami K, Ohtoshi T, Ito K, Satoh M, et al. Increased expression of transforming growth factor-beta1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD). Am J Respir Crit Care Med. 2001. 163:1476–1483.
Article23. Vesey DA, Cheung CW, Cuttle L, Endre ZA, Gobé G, Johnson DW. Interleukin-1beta induces human proximal tubule cell injury, alpha-smooth muscle actin expression and fibronectin production. Kidney Int. 2002. 62:31–40.
Article24. Fan JM, Huang XR, Ng YY, Nikolic-Paterson DJ, Mu W, Atkins RC, et al. Interleukin-1 induces tubular epithelial-myofibroblast transdifferentiation through a transforming growth factor-beta1-dependent mechanism in vitro. Am J Kidney Dis. 2001. 37:820–831.
Article25. Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997. 390:465–471.
Article26. Zavadil J, Bitzer M, Liang D, Yang YC, Massimi A, Kneitz S, et al. Genetic programs of epithelial cell plasticity directed by transforming growth factor-beta. Proc Natl Acad Sci U S A. 2001. 98:6686–6691.
Article27. Tepass U, Truong K, Godt D, Ikura M, Peifer M. Cadherins in embryonic and neural morphogenesis. Nat Rev Mol Cell Biol. 2000. 1:91–100.28. Arias AM. Epithelial mesenchymal interactions in cancer and development. Cell. 2001. 105:425–431.29. Pötter E, Bergwitz C, Brabant G. The cadherin-catenin system: implications for growth and differentiation of endocrine tissues. Endocr Rev. 1999. 20:207–239.
Article30. Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000. 2:76–83.31. Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000. 2:84–89.
Article32. Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, et al. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol cell. 2001. 7:1267–1278.33. Kim K, Lu Z, Hay ED. Direct evidence for a role of beta-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol Int. 2002. 26:463–476.
Article34. Hay ED, Zuk A. Transformations between epithelium and mesenchyme: normal, pathological, and experimentally induced. Am J Kidney Dis. 1995. 26:678–690.
Article35. Hurwitz S, Leiman G, Shapiro C. Mesothelial cells in pleural fluid: TB or not TB? S Afr Med J. 1980. 57:937–939.36. Follador EC, Pimentel M, Barbas CS, Takagaki TY, Kairalla RA, Deheinzelin D, et al. [Tuberculous pleural effusion: clinical and laboratory evaluation]. Rev Hosp Clin Fac Med Sao Paulo. 1991. 46:176–179.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Targeting epithelial-mesenchymal transition pathway in hepatocellular carcinoma
- The Role of Mesothelial Cells in Liver Development, Injury, and Regeneration
- Loosening of the mesothelial barrier as an early therapeutic target to preserve peritoneal function in peritoneal dialysis
- Membrane Proteins Involved in Epithelial-Mesenchymal Transition and Tumor Invasion: Studies on TMPRSS4 and TM4SF5
- Role of Gastric Stem Cells in Gastric Carcinogenesis by Chronic Helicobacter pylori Infection