Korean J Ophthalmol.
2006 Mar;20(1):47-54. 10.3341/kjo.2006.20.1.47.
Characterization of Immortalized Human Corneal Endothelial Cell Line using HPV 16 E6/E7 on Lyophilized Human Amniotic Membrane
- Affiliations
-
- 1Department of Ophthalmology, Chung-Ang University Yongsan Hospital, Seoul, Korea. jck50ey@kornet.net
- 2Department of Chmical and Biochemical Engineering, Dongguk University, Seoul, Korea.
- KMID: 1099072
- DOI: http://doi.org/10.3341/kjo.2006.20.1.47
Abstract
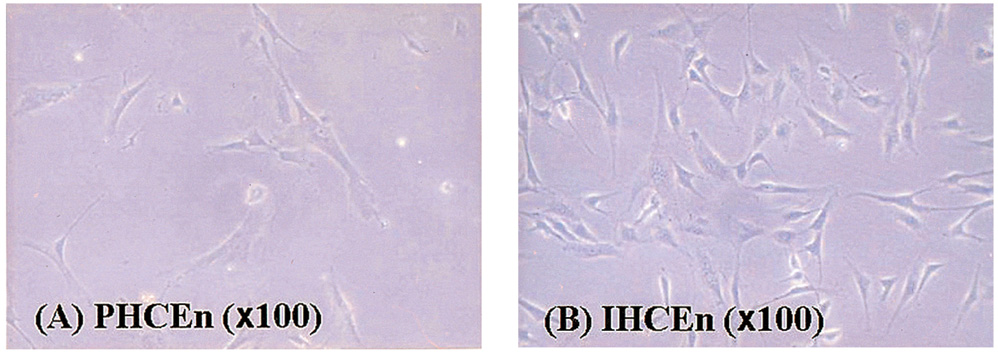
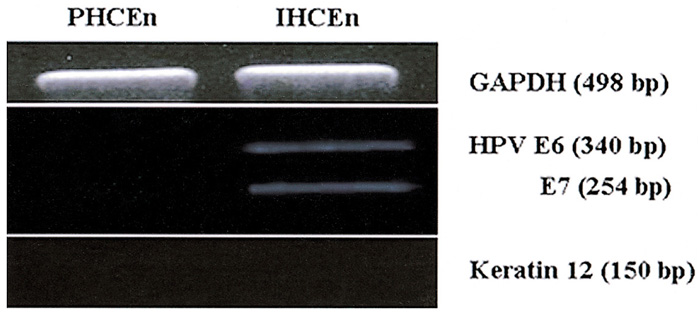
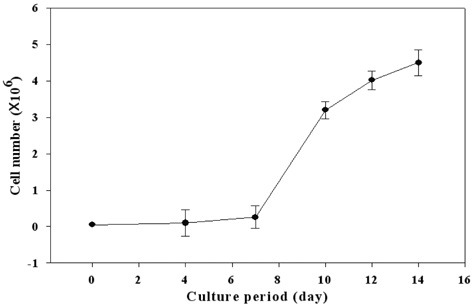
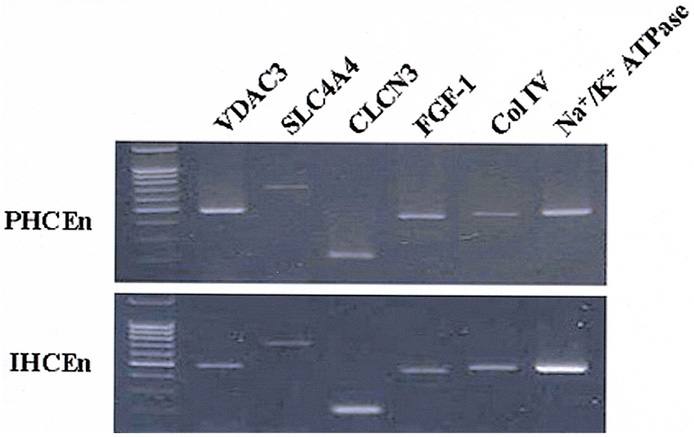
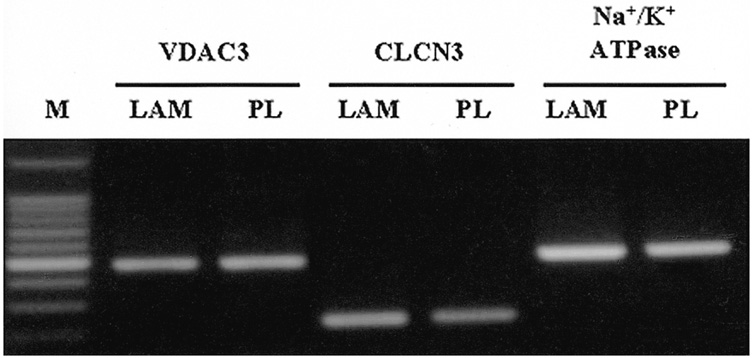
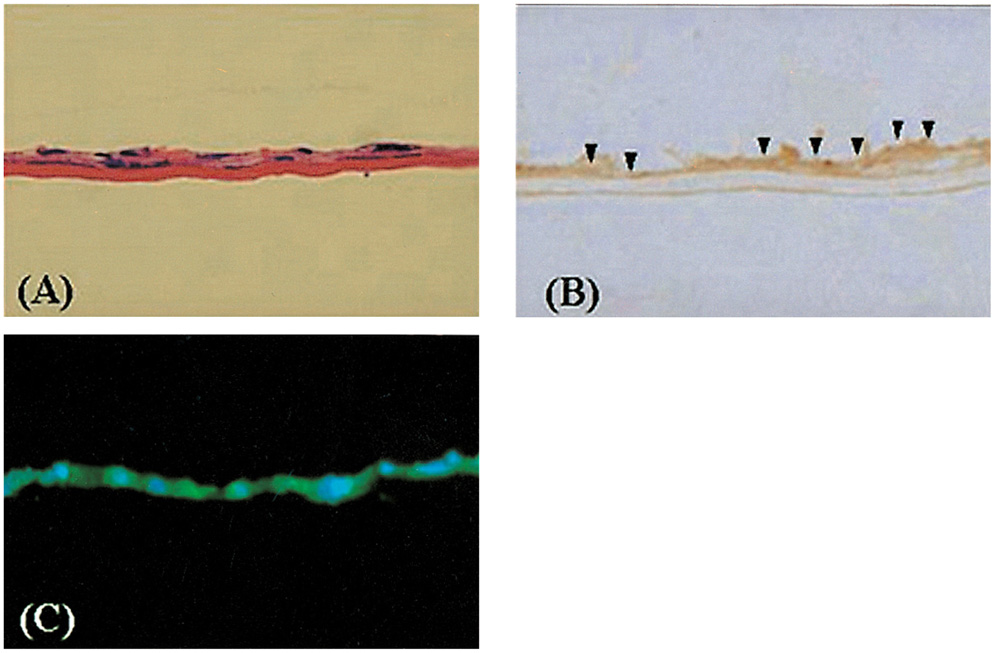
- PURPOSE: To establish the immortalized human corneal endothelial cell line (IHCEn) by transducing human papilloma virus (HPV) 16 E6/E7 oncogenes, and to identify their characteristics when cultivated on a lyophilized human amniotic membrane (LAM). METHODS: Primary human corneal endothelial cells (PHCEn) were infected using a retroviral vector with HPV 16 E6/E7, and transformed cells were clonally selected by G418. Growth properties and characteristics of IHCEn were compared with PHCEn by cell counting and RT-PCR of VDAC3, SLC4A4, CLCN3, FGF-1, Col IV, and Na+/K+ ATPase. IHCEn were cultured on LAM. Messenger RNA expressions of VDAC3, CLCN3, and Na+/K+ ATPase, and protein expressions of Na+/K+ ATPase and Col IV in IHCEn cultivated on LAM were investigated by RT-PCR, immunofluorescence, and immunohistochemical staining, respectively. RESULTS: Successful immortalization was confirmed by stable expression of HPV 16 E6/E7 mRNA by RT-PCR, and IHCEn exhibited typical corneal endothelial morphology. Doubling time of IHCEn was 30.15+/-10.96 hrs. Both IHCEn and PHCEn expressed VDAC3, CLCN3, SLC4A4, FGF-1, Col IV, and Na+/K+ ATPase. IHCEn cultivated on LAM showed stronger expression of VDAC3, CLCN4, and Na+/K+ ATPase mRNA than on plastic culture dish. Immunohistochemical staining and immunofluorescence revealed the positive expression of Na+/K+ ATPase and Col IV. CONCLUSIONS: IHCEn were successfully established, and LAM is a good substrate for the culture of human corneal endothelial cells.
Keyword
MeSH Terms
-
Transfection
Reverse Transcriptase Polymerase Chain Reaction
Repressor Proteins/genetics/*pharmacology
RNA, Messenger/genetics
Protein-Tyrosine Kinases
Oncogene Proteins, Viral/genetics/*pharmacology
Immunohistochemistry
Humans
Gene Expression Regulation, Viral
Freeze Drying
Endothelium, Corneal/*cytology/drug effects/metabolism
Cell Line, Transformed
Cell Count
Amnion
Figure
Reference
-
1. Maurice DM. The location of the fluid pump in the cornea. J Physiol. 1972. 221:43–54.2. Murphy C, Alvarado J, Juster R, et al. Prenatal and postnatal cellularity of the human corneal endothelium. A quantitative histologic study. Invest Ophthalmol Vis Sci. 1984. 25:312–322.3. Bourne WM, Nelson LR, Hodge DO. Central corneal endothelial cell changes over a ten-year period. Invest Ophthalmol Vis Sci. 1997. 38:779–782.4. Bourne WM. Cellular changes in transplanted human corneas. Cornea. 2001. 20:560–569.5. Bourne WM, Kaufman HE. Specular microscopy of human corneal endothelium in vivo. Am J Ophthalmol. 1976. 81:319–323.6. Waring GO 3rd, Bourne WM, Edelhauser HF, et al. The corneal endothelium. Normal and pathologic structure and function. Ophthalmology. 1982. 89:531–590.7. Alvarado JA, Gospodarowicz D, Greeburg G. Corneal endothelial replacement. In vitro formatin of an endothelial monolayer. Invest Ophthalmol Vis Sci. 1981. 21:300–316.8. Gospodarowicz D, Greenburg G. The coating of bovine and rabbit corneas denuded of their endothelium with bovine corneal endothelial cells. Exp Eye Res. 1979. 28:249–265.9. Gospodarowicz D, Greenburg G, Alvarado J. Transplantation of cultured bovine corneal endothelium on rabbit cornea: clinical implication for human studies. Proc Natl Acad Sci. 1979. 76:464.10. Gospodarowicz D, Greenburg G, Alvarado J. Transplantation of cultured bovine corneal endothelium to species with nonregenerative endothelium. Arch Ophthalmol. 1979. 97:2163–2169.11. Insler MS, Lopez JG. Extended incubation times improves corneal endothelial cell transplantation success. Invest Ophthalmol Vis Sci. 1991. 32:1828–1836.12. Joyce NC, Meklir B, Neufeld AH. In vitro pharmacologic separation of corneal endothelial migration and spreading responses. Invest Ophthalmol Vis Sci. 1990. 31:1816–1826.13. Jumblatt MM, Maurice DM, McCulley JP. Transplantation of tissue-cultured corneal endothelium. Invest Ophthalmol Vis Sci. 1978. 17:1135–1141.14. Maurice DM, McCully JP, Perlmn MM. Development in use of cultured endothelium in corneal transplantation. Doc Ophthalmol Proc Ser. 1979. 20:151–153.15. McCulley JP, Maurice DM, Schwartz BD. Corneal endothelial transplantation. Ophthalmology. 1980. 87:194–201.16. Schwartz BD, McCully JP. Morphology of transplanted corneal endothelium derived from tissue culture. Invest Ophthalmol Vis Sci. 1981. 20:467–480.17. Hadlock T, Singh S, Vacanti JP, et al. Ocular cell monolayers cultured on biodegradable substrates. Tissue Engng. 1999. 5:187–196.18. Joo CK, Gree WR, Pepose JS, et al. Repopulation of denude murine Descement's membrane with life-extended murine corneal endothelial cells as a model for corneal transplantation. Graefe's Arch Clin Exp Ophthalmol. 2000. 238:174–180.19. Engelmann K, Bednarz J, Valtink M. Prospects for endothelial transplantation. Exp Eye Res. 2004. 78:573–578.20. Steinberg ML, Defendi V. Transformation and immortalization of human keratinocytes by SV40. J Invest Dermatol. 1983. 81:131S–136S.21. Chang LS, Pan S, Pater MM, et al. Differential requirement for SV40 early genes in immortalization and transformation of primary rat and human embryonic cells. Virology. 1985. 146:246–261.22. Wilson SE, Lloyd SA, He YG, et al. Extended life of human corneal endothelial cells transfected with the SV40 large T antigen. Invest Ophthalmol Vis Sci. 1993. 34:2112–2123.23. Strauss M, Griffin BE. Cellular immortalization-an essential step or merely a risk factor in DNA virus-induced transformation? Cancer Cells. 1990. 2:360–365.24. Wilson SE, Weng J, Blair S, et al. Expression of E6/E7 or SV40 large T antigen-coding oncogenes in human corneal endothelial cells indicates regulated high-proliferative capacity. Invest Ophthalmol Vis Sci. 1995. 36:32–40.25. Fehrmann F, Laimins LA. Human papillomaviruses: targeting differentiating epithelial cells for malignant transformation. Oncogene. 2003. 22:5201–5207.26. Shin JS, Jang IK, Kim CH, et al. Development and characterization of a rabbit corneal endothelial cell line. Jpn J Ophthalmol. 2004. 48:454–459.27. Wilson SE, Weng J, Blair S, et al. Expression of E6/E7 or SV40 large T antigen-coding oncogenes in human corneal endothelial cells indicates regulated high-proliferative capacity. Invest Ophthalmol Vis Sci. 1995. 36:32–40.28. He YG, Weng J, Li Q, et al. Fuch's corneal endothelial cells transduced with the human papilloma virus E6/E7 oncogenes. Exp Eye Res. 1997. 65:135–142.29. Fehrmann F, Laimins LA. Human papillomaviruses: targeting differentiating epithelial cells for malignant transformation. Oncogene. 2003. 22:5201–5207.30. Vousden KH. Interactions between papillomavirus proteins and tumor suppressor gene products. Adv Cancer Res. 1994. 64:1–24.31. Bedell MA, Jones KH, Grossman SR, et al. Identification of human papillomavirus type 18 transforming genes in immortalized and primary cells. J Virol. 1989. 63:1247–1255.32. Halbert CL, Demers GW, Galloway DA. The E7 gene of human papillomavirus type 16 is sufficient for immortalization of human epithelial cells. J Virol. 1991. 65:473–478.33. Cole ST, Danos O. Nucleotide sequence and comparative analysis of the human papillomavirus type 18 genome. Phylogeny of papillomaviruses and repeated structure of the E6 and E7 gene products. J Mol Biol. 1987. 193:599–608.34. Wigham CG, Turner HC, Swan J, et al. Modulation of corneal endothelial hydration control mechanisms by Rolipram. Pflugers Arch. 2000. 440:866–870.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of cell growth suppression in SiHa cervical carcinoma cell line by human papillomavirus type 16 E6/E7 siRNAs
- Expression and localization of human papillomavirus type 16 E6 and E7 open reading frame proteins in human epidermal keratinocyte
- Human Papillomavirus Infection
- Improved immunodetection of human papillomavirus E7
- Establishment of Immotalized Human Gingival Fibroblast Cell Lines