J Korean Med Sci.
2005 Apr;20(2):242-247. 10.3346/jkms.2005.20.2.242.
Promoter Methylation of E-cadherin in Hepatocellular Carcinomas and Dysplastic Nodules
- Affiliations
-
- 1Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. ckpark@smc.samsung.co.kr
- 2Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of General Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Department of Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 1095198
- DOI: http://doi.org/10.3346/jkms.2005.20.2.242
Abstract
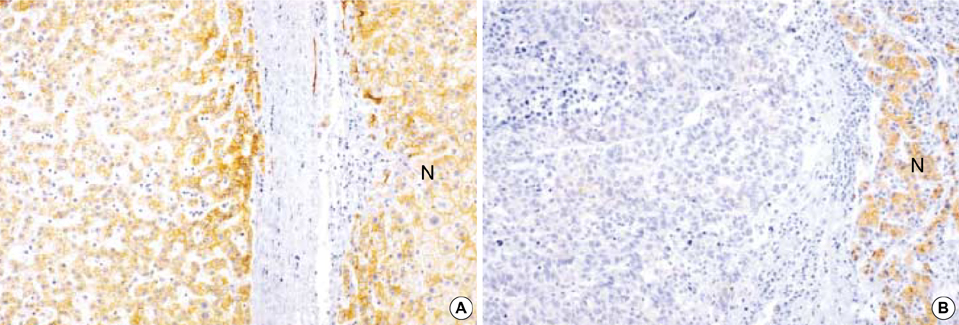
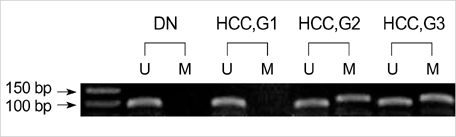
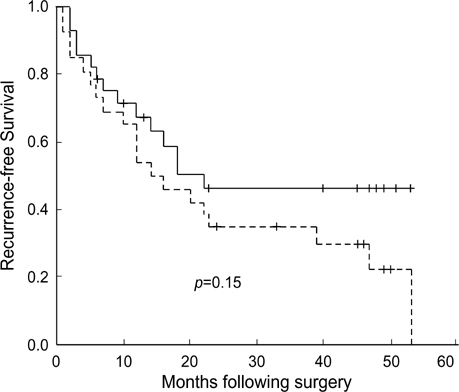
- In order to clarify the significance of E-cadherin methylation in multistep hepatocarcinogenesis, we examined the methylation status of the E-cadherin promoter region, using methylation-specific polymerase chain reaction in 64 hepatocellular carcinomas (HCCs) and 13 dysplastic nodules (DNs), and correlated these results with E-cadherin protein expression and clinicopathologic factors of HCCs. Promoter methylation was detected in 1 of 13 (7.7%) DNs, in 5 of 13 (38.5%) Edmondson and Steiner grade I HCCs, and in 27 of 51 (52.9%) grade II or III HCCs, and a significant correlation was observed between the methylation status and the stepwise progression of hepatocarcinogenesis (p=0.004). Reduced E-cadherin immunoreactivity was found in 18 of 64 (28%) HCCs, but in none of DNs. E-cadherin methylation status in HCCs was significantly correlated with microvascular invasion (p=0.02) and tumor recurrence (p=0.04), but not with reduced E-cadherin immunoreactivity. The Kaplan-Meier method showed that methylation status did not have a significant influence on the recurrence-free survival of HCC patients (p=0.15). Our results indicate that methylation of the E-cadherin promoter region is a frequent event in HCC, which may play an important role in the stepwise progression of hepatocarcinogenesis. And the promoter methylation of E-cadherin in HCC was found to be significantly correlated with microvascular invasion and recurrence.
MeSH Terms
Figure
Cited by 1 articles
-
Clinicopathologic Correlations of E-cadherin and Prrx-1 Expression Loss in Hepatocellular Carcinoma
Kijong Yi, Hyunsung Kim, Yumin Chung, Hyein Ahn, Jongmin Sim, Young Chan Wi, Ju Yeon Pyo, Young-Soo Song, Seung Sam Paik, Young-Ha Oh
J Pathol Transl Med. 2016;50(5):327-336. doi: 10.4132/jptm.2016.06.22.
Reference
-
1. Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991. 251:1451–1455.
Article2. Mareel M, Boterberg T, Noe V, Van Hoorde L, Vermeulen S, Bruyneel E, Bracke M. E-cadherin/catenin/cytoskeleton complex: a regulator of cancer invasion. J Cell Physiol. 1997. 173:271–274.
Article3. Huang GT, Lee HS, Chen CH, Sheu JC, Chiou LL, Chen DS. Correlation of E-cadherin expression and recurrence of hepatocellular carcinoma. Hepatogastroenterology. 1999. 46:1923–1927.4. Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, Hofler H. E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res. 1994. 54:3845–3852.5. Berx G, Cleton-Jansen AM, Nollet F, de Leeuw WJ, van de Vijver M, Cornelisse C, van Roy F. E-cadherin is a tumour invasion suppressor gene mutated in human lobular breast cancers. EMBO J. 1995. 14:6107–6115.6. Matsumura T, Makino R, Mitamura K. Frequent down-regulation of E-cadherin by genetic and epigenetic changes in the malignant progression of hepatocellular carcinomas. Clin Cancer Res. 2001. 7:594–599.7. Kanai Y, Ushijima S, Hui AM, Ochiai A, Tsuda H, Sakamoto M, Hirohashi S. The E-cadherin gene is silenced by CpG methylation in human hepatocellular carcinomas. Int J Cancer. 1997. 71:355–359.
Article8. Shen JC, Rideout WM 3rd, Jones PA. High frequency mutagenesis by a DNA methyltransferase. Cell. 1992. 71:1073–1080.
Article9. Kanai Y, Ushijima S, Tsuda H, Sakamoto M, Sugimura T, Hirohashi S. Aberrant DNA methylation on chromosome 16 is an early event in hepatocarcinogenesis. Jpn J Cancer Res. 1996. 87:1210–1217.
Article10. Li E, Beard C, Jaenisch R. Role for DNA methylation in genomic imprinting. Nature. 1993. 366:362–365.
Article11. Herman JG. Hypermethylation of tumor suppressor genes in cancer. Semin Cancer Biol. 1999. 9:359–367.
Article12. Yoshiura K, Kanai Y, Ochiai A, Shimoyama Y, Sugimura T, Hirohashi S. Silencing of the E-cadherin invasion-suppressor gene by CpG methylation in human carcinomas. Proc Natl Acad Sci USA. 1995. 92:7416–7419.
Article13. Wei Y, van Nhieu JT, Prigent S, Srivatanakul P, Tiollais P, Buendia MA. Altered expression of E-cadherin in hepatocellular carcinoma: correlation with genetic alterations, beta-catenin expression, and clinical features. Hepatology. 2002. 36:692–701.14. International Working Party. Terminology of nodular hepatocellular lesions. Hepatology. 1995. 22:983–993.15. Lee S, Lee HJ, Kim JH, Lee HS, Jang JJ, Kang GH. Aberrant CpG island hypermethylation along multistep hepatocarcinogenesis. Am J Pathol. 2003. 163:1371–1378.
Article16. Edmondson HA, Steiner PE. Primary carcinoma of the liver. A study of 100 cases among 48900 necropsies. Cancer. 1954. 7:462–503.17. Greene FL, Page DL, Fleming ID. AJCC cancer staging manual. 2002. 6th ed. New York: Springer;132–138.18. Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996. 93:9821–9826.
Article19. Kanai Y, Ushijima S, Tsuda H, Sakamoto M, Hirohashi S. Aberrant DNA methylation precedes loss of heterozygosity on chromosome 16 in chronic hepatitis and liver cirrhosis. Cancer Lett. 2000. 148:73–80.
Article20. Yang B, Guo M, Herman JG, Clark DP. Aberrant promotor methylation profiles of tumor suppressor genes in hepatocellular carcinoma. Am J Pathol. 2003. 163:1101–1107.21. Graff JR, Gabrielson E, Fujii H, Baylin SB, Herman JG. Methylation patterns of the E-cadherin 5' CpG island are unstable and reflect the dynamic, heterogeneous loss of E-cadherin expression during metastatic progression. J Biol Chem. 2000. 275:2727–2732.
Article22. Shiozaki H, Tahara H, Oka H, Miyata M, Kobayashi K, Tamura S, Iihara K, Doki Y, Hirano S, Takeichi M, Mori T. Expression of immunoreactive E-cadherin adhesion molecules in human cancers. Am J Pathol. 1991. 139:17–23.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hypermethylation of E-cadherin in endometrial carcinoma
- Loss of E-cadherin Function is Suggested to be Associated with Peritoneal Seeding in Colorectal Cancer
- Analysis of Epigenetic Marker of Bladder Cancer
- Expression of Glutathione S-Transferase, E-Cadherin, and Catenins during N,N-Diethylnitrosamine-Induced Hepatocarcinogenesis in Rat Liver
- Pathology of Hepatocellular Carcinoma: Recent Update