Yonsei Med J.
2007 Feb;48(1):41-47. 10.3349/ymj.2007.48.1.41.
Antiallodynic Effect of Pregabalin in Rat Models of Sympathetically Maintained and Sympathetic Independent Neuropathic Pain
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine and Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Seoul, Korea. ywleepain@yumc.yonsei.ac.kr
- KMID: 1093522
- DOI: http://doi.org/10.3349/ymj.2007.48.1.41
Abstract
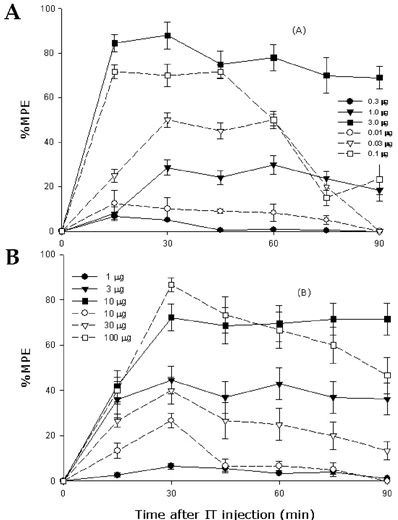
- Pregabalin binds to the voltage-dependent calcium channel alphadelta subunit and modulates the release of neurotransmitters, resulting in analgesic effects on neuropathic pain. Neuropathic pain has both sympathetically maintained pain (SMP) and sympathetic independent pain (SIP) components. We studied the antiallodynic effects of pregabalin on tactile allodynia (TA) and cold allodynia (CA) in SMP-and SIP-dominant neuropathic pain models. Allodynia was induced by ligation of the L5 & L6 spinal nerves (SMP model) or by transection of the tibial and sural nerves (SIP model) in rats. For intrathecal drug administration, a PE-10 catheter was implanted through the atlantooccipital membrane to the lumbar enlargement. Pregabalin was administered either intraperitoneally (IP) or intrathecally (IT) and dosed up incrementally until an antiallodynic effect without sedation or motor impairment was apparent. TA was assessed using von Frey filaments, and CA was assessed using acetone drops. IP-administered pregabalin dose-dependently attenuated TA in both models and CA in the SMP model, but not CA in the SIP model. IT-administered pregabalin dose-dependently attenuated both TA and CA in both models. However, the dose response curve of IT-administered pregabalin in SMP was shifted to left from that of SIP and the ED50 of IT-administered pregabalin for CA in SMP was about 900 times less than that in SIP. These findings suggest that pregabalin exerts its antiallodynic effect mainly by acting at the spinal cord, and that IT-administered pregabalin has more potent antiallodynic effects in SMP. The alphadeltasubunit might be less involved in the CA in SIP.
Keyword
Figure
Reference
-
1. Attal N, Bouhassira D. Mechanisms of pain in peripheral neuropathy. Acta Neurol Scand Suppl. 1999. 173:12–24.
Article2. Kim SH, Na HS, Sheen K, Chung JM. Effects of sympathectomy on a rat model of peripheral neuropathy. Pain. 1993. 55:85–92.
Article3. Lee BH, Won R, Baik EJ, Lee SH, Moon CH. An animal model of neuropathic pain employing injury to the sciatic nerve branches. Neuroreport. 2000. 11:657–661.
Article4. Han DW, Kweon TD, Kim KJ, Lee JS, Chang CH, Lee YW. Does the tibial and sural nerve transection model represent sympathetically independent pain? Yonsei Med J. 2006. 47:847–851.
Article5. Klugbauer N, Marais E, Hofmann F. Calcium channel alpha2delta subunits: differential expression, function, and drug binding. J Bioenerg Biomembr. 2003. 35:639–647.
Article6. Stahl SM. Mechanism of action of alpha2delta ligands: voltage sensitive calcium channel (VSCC) modulators. J Clin Psychiatry. 2004. 65:1033–1034.
Article7. Fink K, Dooley DJ, Meder WP, Suman-Chauhan N, Duffy S, Clusmann H, et al. Inhibition of neuronal Ca(2+) influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology. 2002. 42:229–236.
Article8. Dooley DJ, Mieske CA, Borosky SA. Inhibition of K(+)-evoked glutamate release from rat neocortical and hippocampal slices by gabapentin. Neurosci Lett. 2000. 280:107–110.
Article9. Field MJ, McCleary S, Hughes J, Singh L. Gabapentin and pregabalin, but not morphine and amitriptyline, block both static and dynamic components of mechanical allodynia induced by streptozocin in the rat. Pain. 1999. 80:391–398.
Article10. Field MJ, Oles RJ, Lewis AS, McCleary S, Hughes J, Singh L. Gabapentin (neurontin) and S-(+)-3-isobutylgaba represent a novel class of selective antihyperalgesic agents. Br J Pharmacol. 1997. 121:1513–1522.
Article11. Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994. 59:369–376.
Article12. Luo ZD, Chaplan SR, Higuera ES, Sorkin LS, Stauderman KA, Williams ME, et al. Upregulation of dorsal root ganglion (alpha)2(delta) calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J Neurosci. 2001. 21:1868–1875.
Article13. Hwang JH, Yaksh TL. Effect of subarachnoid gabapentin on tactile-evoked allodynia in a surgically induced neuropathic pain model in the rat. Reg Anesth. 1997. 22:249–256.
Article14. Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992. 50:355–363.
Article15. Chung JM, Choi Y, Yoon YW, Na HS. Effects of age on behavioral signs of neuropathic pain in an experimental rat model. Neurosci Lett. 1995. 183:54–57.
Article16. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994. 53:55–63.
Article17. Tolle TR, Berthele A, Schadrack J, Zieglgansberger W. Involvement of glutamatergic neurotransmission and protein kinase C in spinal plasticity and the development of chronic pain. Prog Brain Res. 1996. 110:193–206.18. Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999. 353:1959–1964.
Article19. Takahashi K, Sato J, Mizumura K. Responses of C-fiber low threshold mechanoreceptors and nociceptors to cold were facilitated in rats persistently inflamed and hypersensitive to cold. Neurosci Res. 2003. 47:409–419.
Article20. Wasner G, Schattschneider J, Binder A, Baron R. Topical menthol-a human model for cold pain by activation and sensitization of C nociceptors. Brain. 2004. 127:1159–1171.
Article21. Kim YI, Kim SH, Oh EJ, Sung B, Na HS, Han HC, et al. Some membrane property changes following axotomy in A delta-type DRG cells are related to cold allodynia in rat. Neuroreport. 1999. 10:1493–1499.
Article22. Nozaki-Taguchi N, Yaksh TL. Pharmacology of spinal glutamatergic receptors in post-thermal injury-evoked tactile allodynia and thermal hyperalgesia. Anesthesiology. 2002. 96:617–626.
Article23. Bian F, Li Z, Offord J, Davis MD, McCormick J, Taylor CP, et al. Calcium channel alpha2-delta type 1 subunit is the major binding protein for pregabalin in neocortex, hippocampus, amygdala, and spinal cord: an ex vivo autoradiographic study in alpha2-delta type 1 genetically modified mice. Brain Res. 2006. 1075:68–80.
Article24. Li CY, Song YH, Higuera ES, Luo ZD. Spinal dorsal horn calcium channel alpha2delta-1 subunit upregulation contributes to peripheral nerve injury-induced tactile allodynia. J Neurosci. 2004. 24:8494–8499.
Article25. Shimoyama M, Shimoyama N, Hori Y. Gabapentin affects glutamatergic excitatory neurotransmission in the rat dorsal horn. Pain. 2000. 85:405–414.
Article26. Wu WP, Hao JX, Ongini E, Impagnatiello F, Presotto C, Wiesenfeld-Hallin Z, et al. A nitric oxide (NO)-releasing derivative of gabapentin, NCX 8001, alleviates neuropathic pain-like behavior after spinal cord and peripheral nerve injury. Br J Pharmacol. 2004. 141:65–74.
Article27. Luo ZD, Calcutt NA, Higuera ES, Valder CR, Song YH, Svensson CI, et al. Injury type-specific calcium channel alpha 2 delta-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J Pharmacol Exp Ther. 2002. 303:1199–1205.
Article28. Kayser V, Christensen D. Antinociceptive effect of systemic gabapentin in mononeuropathic rats, depends on stimulus characteristics and level of test integration. Pain. 2000. 88:53–60.
Article29. Taylor CP, Vartanian MG, Yuen PW, Bigge C, Suman-Chauhan N, Hill DR. Potent and stereospecific anticonvulsant activity of 3-isobutyl GABA relates to in vitro binding at a novel site labeled by tritiated gabapentin. Epilepsy Res. 1993. 14:11–15.
Article30. Chen SR, Xu Z, Pan HL. Stereospecific effect of pregabalin on ectopic afferent discharges and neuropathic pain induced by sciatic nerve ligation in rats. Anesthesiology. 2001. 95:1473–1479.
Article31. Takahashi N, Kikuchi S, Dai Y, Kobayashi K, Fukuoka T, Noguchi K. Expression of auxiliary beta subunits of sodium channels in primary afferent neurons and the effect of nerve injury. Neuroscience. 2003. 121:441–450.
Article32. McLachlan EM, Janig W, Devor M, Michaelis M. Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature. 1993. 363:543–546.
Article33. Ali Z, Ringkamp M, Hartke TV, Chien HF, Flavahan NA, Campbell JN, et al. Uninjured C-fiber nociceptors develop spontaneous activity and alpha-adrenergic sensitivity following L6 spinal nerve ligation in monkey. J Neurophysiol. 1999. 81:455–466.
Article34. Sluka KA. Blockade of calcium channels can prevent the onset of secondary hyperalgesia and allodynia induced by intradermal injection of capsaicin in rats. Pain. 1997. 71:157–164.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Does the Tibial and Sural Nerve Transection Model Represent Sympathetically Independent Pain?
- Effects of Clonidine and Epinephrine on Neuropathic Pain in an Experimental Animal Model for Peripheral Neuropathy
- Pharmacological interactions between intrathecal pregabalin plus tianeptine or clopidogrel in a rat model of neuropathic pain
- The Combined Antiallodynic Effect of Gabapentin and Milnacipran in a Rat Neuropathic Pain Model
- The Effect of MK-801 and Naloxone in a Sympathetically Independent Neuropathic Pain Rat Model