Korean J Radiol.
2006 Dec;7(4):249-256. 10.3348/kjr.2006.7.4.249.
Comparison of CT and 18F-FDG PET for Detecting Peritoneal Metastasis on the Preoperative Evaluation for Gastric Carcinoma
- Affiliations
-
- 1Department of Diagnostic Radiology, Yonsei University College of Medicine, Seoul, Korea. kimnex@yumc.yonsei.ac.kr
- 2Institute of Gastroenterology, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Nuclear Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Nuclear Medicine, Hallym University College of Medicine, Anyang, Korea.
- 5Department of Surgery, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1092548
- DOI: http://doi.org/10.3348/kjr.2006.7.4.249
Abstract
OBJECTIVE
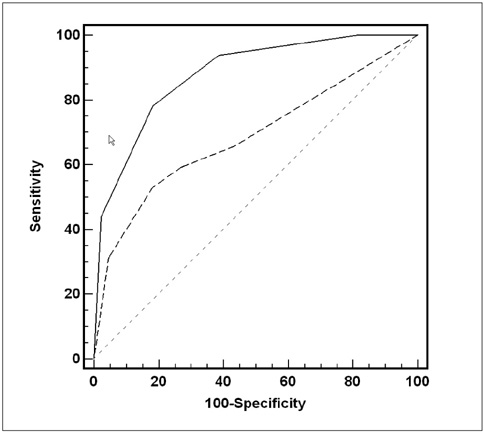
The aim of our study was to compare the accuracy of CT and 18F-FDG PET for detecting peritoneal metastasis in patients with gastric carcinoma. MATERIALS AND METHODS: One-hundred-twelve patients who underwent a histologic confirmative exam or treatment (laparotomy, n = 107; diagnostic laparoscopy, n = 4; peritoneal washing cytology, n = 1) were retrospectively enrolled. All the patients underwent CT and 18F-FDG PET scanning for their preoperative evaluation. The sensitivities, specificities and accuracies of CT and 18F-FDG PET imaging for the detection of peritoneal metastasis were calculated and then compared using Fisher's exact probability test (p < 0.05), on the basis of the original preoperative reports. In addition, two board-certified radiologists and two board-certified nuclear medicine physicians independently reviewed the CT and PET scans, respectively. A receiver-operating characteristic curve analysis was performed to compare the diagnostic performance of CT and 18F-FDG PET imaging for detecting peritoneal metastasis. RESULTS: Based on the original preoperative reports, CT and 18F-FDG PET showed sensitivities of 76.5% and 35.3% (p = 0.037), specificities of 91.6% and 98.9% (p = 0.035), respectively, and equal accuracies of 89.3% (p = 1.0). The receptor operating characteristics curve analysis showed a significantly higher diagnostic performance for CT (Az = 0.878) than for PET (Az = 0.686) (p = 0.004). The interobserver agreement for detecting peritoneal metastasis was good (κ value = 0.684) for CT and moderate (κ value = 0.460) for PET. CONCLUSION: For the detection of peritoneal metastasis, CT was more sensitive and showed a higher diagnostic performance than PET, although CT had a relatively lower specificity than did PET.
Keyword
MeSH Terms
-
*Tomography, Emission-Computed
Stomach Neoplasms/*pathology
Sensitivity and Specificity
Retrospective Studies
Radiopharmaceuticals/diagnostic use
ROC Curve
*Positron-Emission Tomography
Peritoneal Neoplasms/*radiography/*radionuclide imaging/*secondary
Middle Aged
Male
Iohexol/analogs & derivatives/diagnostic use
Humans
Fluorodeoxyglucose F18/diagnostic use
Female
Contrast Media
Aged, 80 and over
Aged
Adult
Adolescent
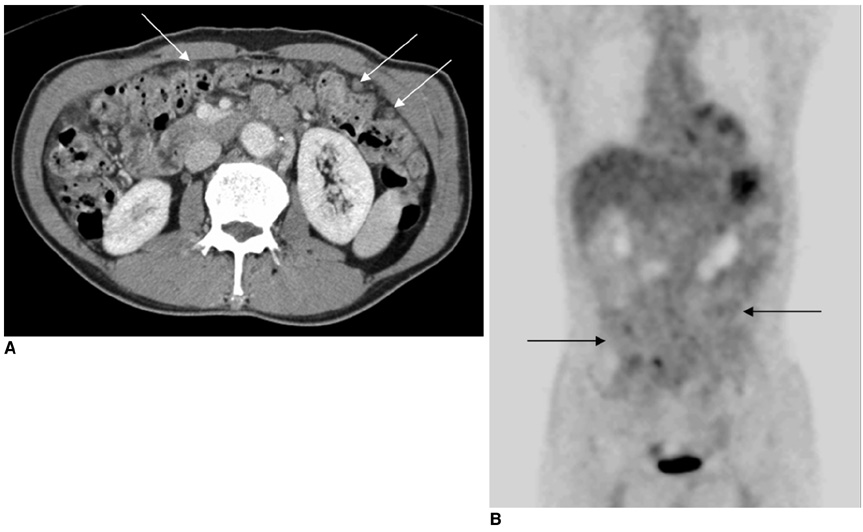
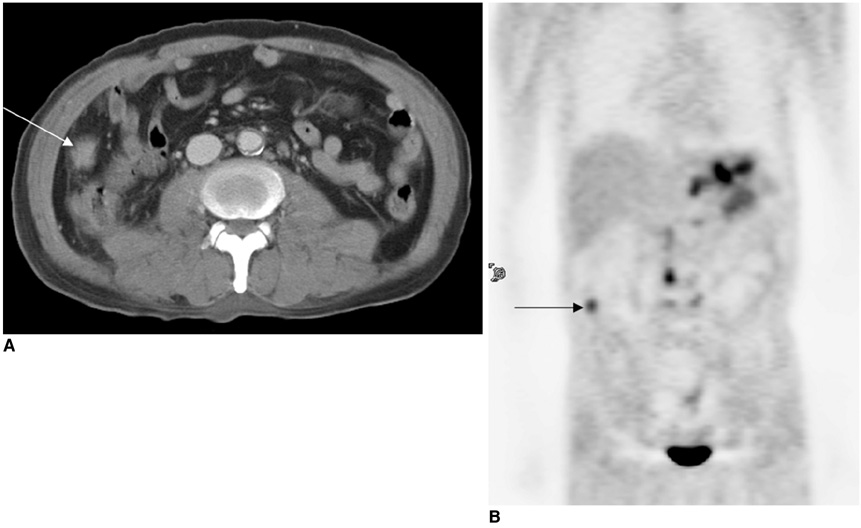
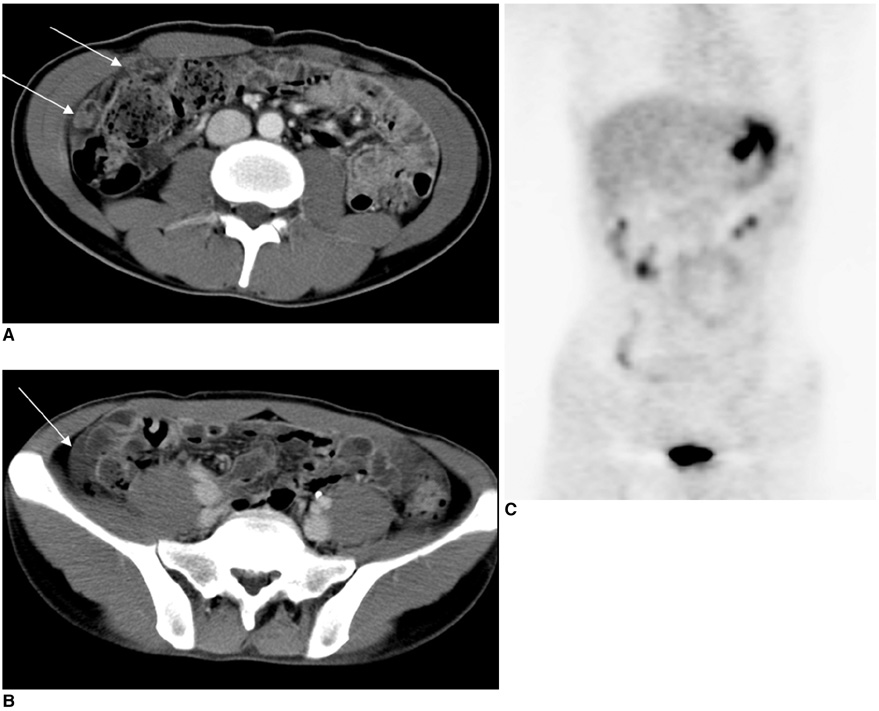
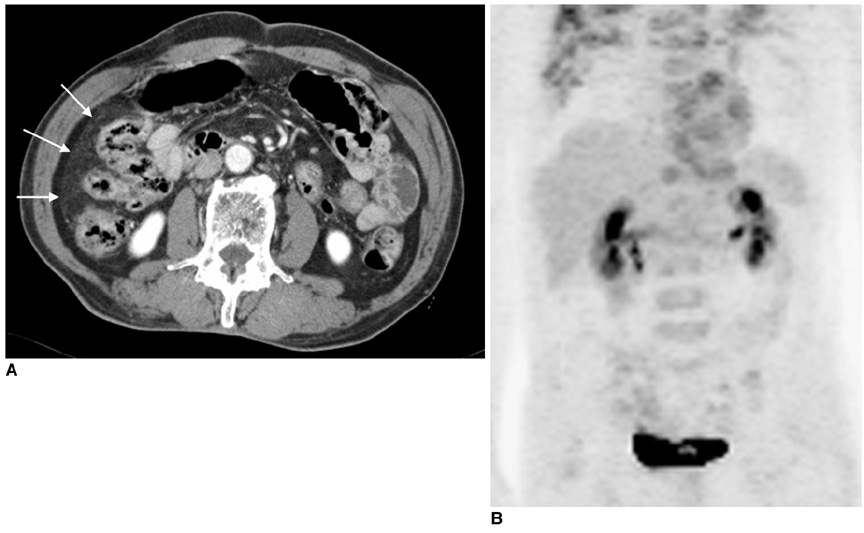
Figure
Cited by 3 articles
-
Imaging of Gastric Cancer Metabolism Using 18 F-FDG PET/CT
Mijin Yun
J Gastric Cancer. 2014;14(1):1-6. doi: 10.5230/jgc.2014.14.1.1.Role of 18F 2-fluoro-2-deoxyglucose Positron Emission Tomography in Upper Gastrointestinal Malignancies
Hye-Won Yun, Ki-Nam Shim
Korean J Gastroenterol. 2013;61(6):303-306. doi: 10.4166/kjg.2013.61.6.303.Diagnostic performance of F-18 FDG PET or PET/CT for detection of recurrent gastric cancer: a systematic review and meta-analysis
Chang In Choi, Jae Kyun Park, Tae Yong Jeon, Dae-Hwan Kim
J Yeungnam Med Sci. 2023;40(Suppl):S37-S46. doi: 10.12701/jyms.2023.00220.
Reference
-
1. Champault G, Barrat C. Laparoscopy in the staging of cancer of the stomach. J Chir (Paris). 1999. 136:150–155.2. Davies J, Chalmers AG, Sue-Ling HM, May J, Miller GV, Martin IG, et al. Spiral computed tomography and operative staging of gastric carcinoma: a comparison with histopathological staging. Gut. 1997. 41:314–319.3. Dux M, Richter GM, Hansmann J, Kuntz C, Kauffmann GW. Helical hydro-CT for diagnosis and staging of gastric carcinoma. J Comput Assist Tomogr. 1999. 23:913–922.4. Sendler A, Dittler HJ, Feussner H, Nekarda H, Bollschweiler E, Fink U, et al. Preoperative staging of gastric cancer as precondition for multimodal treatment. World J Surg. 1995. 19:501–508.5. Low RN, Barone RM, Lacey C, Sigeti JS, Alzate GD, Sebrechts CP. Peritoneal tumor: MR imaging with dilute oral barium and intravenous gadolinium-containing contrast agents compared with unenhanced MR imaging and CT. Radiology. 1997. 204:513–520.6. Gryspeerdt S, Clabout L, Van Hoe L, Berteloot P, Vergote IB. Intraperitoneal contrast material combined with CT for detection of peritoneal metastases of ovarian cancer. Eur J Gynaecol Oncol. 1998. 19:434–437.7. Boudiaf M, Bedda S, Soyer P, Panis Y, Zidi S, Kardache M, et al. Preoperative evaluation of gastric adenocarcinomas: comparison of CT results with surgical and patholgic results. Ann Chir. 1999. 53:115–122.8. Rohren EM, Turkington TG, Coleman RE. Clinical applications of PET in oncology. Radiology. 2004. 231:305–332.9. Park DH, Kim KH, Park SY, Lee BH, Choi CW, Chin SY. Diagnosis of recurrent uterine cervical cancer: computed tomography versus positron emission tomography. Korean J Radiol. 2000. 1:51–55.10. Kluge R, Schmidt F, Caca K, Barthel H, Hesse S, Georgi P, et al. Positron emission tomography with [(18)F]fluoro-2-deoxy-D-glucose for diagnosis and staging of bile duct cancer. Hepatology. 2001. 33:1029–1035.11. Tanaka T, Kawai Y, Kanai M, Taki Y, Nakamoto Y, Takabayashi A. Usefulness of FDG-positron emission tomography in diagnosing peritoneal recurrence of colorectal cancer. Am J Surg. 2002. 184:433–436.12. Turlakow A, Yeung HW, Salmon AS, Macapinlac HA, Larson SM. Peritoneal carcinomatosis: role of (18)F-FDG PET. J Nucl Med. 2003. 44:1407–1412.13. Buy JN, Moss AA, Ghossain MA, Sciot C, Malbec L, Vadrot D, et al. Peritoneal implants from ovarian tumors: CT findings. Radiology. 1988. 169:691–694.14. Halvorsen RA Jr, Panushka C, Oakley GJ, Letourneau JG, Adcock LL. Intraperitoneal contrast material improves the CT detection of peritoneal metastases. AJR Am J Roentgenol. 1991. 157:37–40.15. Walkey MM, Friedman AC, Sohotra P, Radecki PD. CT manifestations of peritoneal carcinomatosis. AJR Am J Roentgenol. 1988. 150:1035–1041.16. Coakley FV, Choi PH, Gougoutas CA, Pothuri B, Venkatraman E, Chi D, et al. Peritoneal metastases: detection with spiral CT in patients with ovarian cancer. Radiology. 2002. 223:495–499.17. Lin EC, Lear J, Quaife RA. Metastatic peritoneal seeding patterns demonstrated by FDG positron emission tomographic imaging. Clin Nucl Med. 2001. 26:249–250.18. Fleiss J. Statistical methods for rates and proportions. 1981. 2nd ed. New York: Wiley;212–236.19. Tang Y, Yamashita Y, Arakawa A, Namimoto T, Mitsuzaki K, Abe Y, et al. Detection of hepatocellular carcinoma arising in cirrhotic livers: comparison of gadolinium- and ferumoxides-enhanced MR imaging. AJR Am J Roentgenol. 1999. 172:1547–1554.20. Blakeborough A, Ward J, Wilson D, Griffiths M, Kajiya Y, Guthrie JA, et al. Hepatic lesion detection at MR imaging: a comparative study with four sequences. Radiology. 1997. 203:759–765.21. Wang PH, Liu RS, Li YF, Ng HT, Yuan CC. Whole-body PET with (fluorine-18)-2-deoxyglucose for detecting recurrent primary serous peritoneal carcinoma: An initial report. Gynecol Oncol. 2000. 77:44–47.22. Sussman SK, Halvorsen RA Jr, Illescas FF, Cohan RH, Saeed M, Silverman PM, et al. Gastric adenocarcinoma: CT versus surgical staging. Radiology. 1988. 167:335–340.23. Prayer L, Kainz C, Kramer J, Stiglbauer R, Schurawitzki H, Baldt M, et al. CT and MR accuracy in the detection of tumor recurrence in patients treated for ovarian cancer. J Comput Assist Tomogr. 1993. 17:626–632.24. Stahl A, Ott K, Weber WA, Becker K, Link T, Siewert JR, et al. FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging. 2003. 30:288–295.25. Yoshioka T, Yamaguchi K, Kubota K, Saginoya T, Yamazaki T, Ido T, et al. Evaluation of 18F-FDG PET in patients with a, metastatic, or recurrent gastric cancer. J Nucl Med. 2003. 44:690–699.26. Ming SC. Gastric carcinoma. A pathobiological classification. Cancer. 1977. 39:2475–2285.27. Noda S, Soejima K, Inokuchi K. Clinicopathological analysis of the intestinal type and diffuse type of gastric carcinoma. Jpn J Surg. 1980. 10:277–283.28. Sugano H, Nakamura K, Kato Y. Pathological studies of human gastric cancer. Acta Pathol Jpn. 1982. 32:Suppl 2. 329–347.29. De Potter T, Flamen P, Van Cutsem E, Penninckx F, Filez L, Bormans G, et al. Whole-body PET with FDG for the diagnosis of recurrent gastric cancer. Eur J Nucl Med Mol Imaging. 2002. 29:525–529.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- F18-fluorodeoxyglucose-positron emission tomography and computed tomography is not accurate in preoperative staging of gastric cancer
- The Diagnostic Utility of PET-CT for the Preoperative Evaluation of Lymph Node Metastasis in Gastric Cancer Patients
- Use of 18F-FDG PET/CT in Second Primary Cancer
- Late Port Site Metastasis from Occult Gall Bladder Carcinoma After Laparoscopic Cholecystectomy for Cholelithiasis: The Role of 18F-FDG PET/CT
- Supraclavicular Lymph Node Metastasis from Various Malignancies: Assessment with 18F-Fluorodeoxyglucose Positron Emission Tomography/CT, Contrast-Enhanced CT and Ultrasound