J Vet Sci.
2010 Dec;11(4):305-313. 10.4142/jvs.2010.11.4.305.
Immunomodulatory effects of betulinic acid from the bark of white birch on mice
- Affiliations
-
- 1College of Animal Medicine, Hunan Agricultural University, Changsha, 410128, P.R. China. yuanhui7269@yahoo.com.cn
- 2Department of Biochemistry, Pharmacology and Toxicology, Faculty of Veterinary Medicine, Wroclaw University of Environment and Life Science, Norwida 31, 50-375, Wroclaw, Poland.
- KMID: 1072179
- DOI: http://doi.org/10.4142/jvs.2010.11.4.305
Abstract
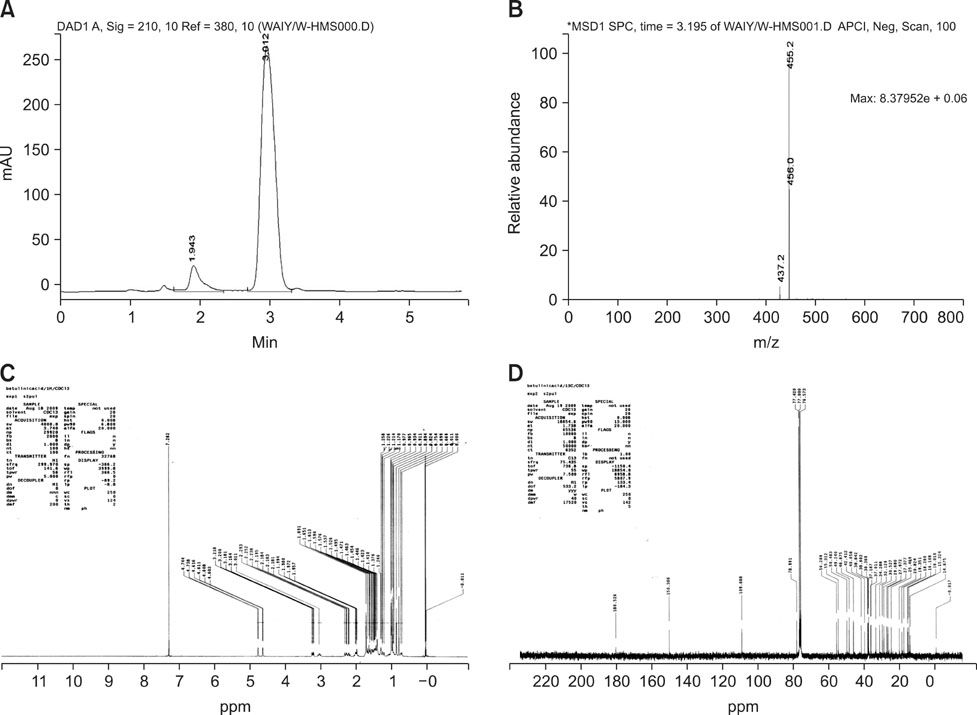
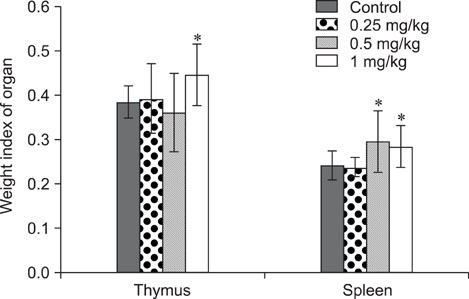
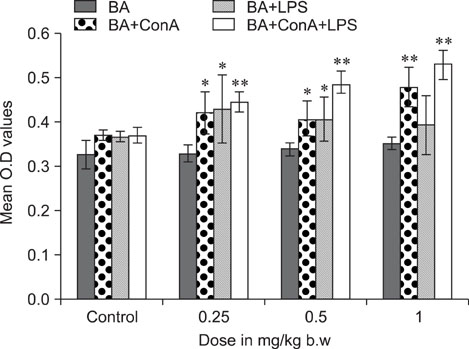
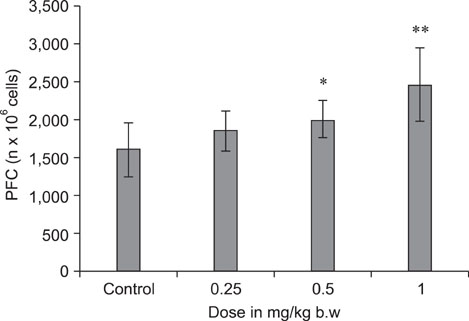
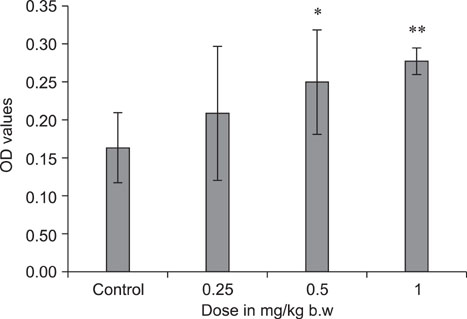
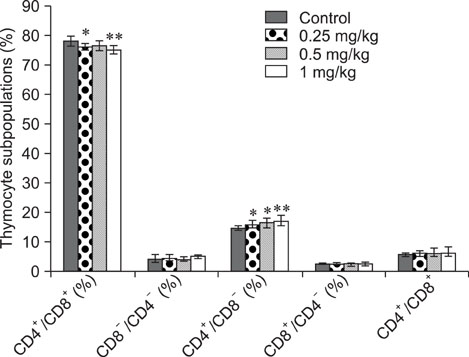
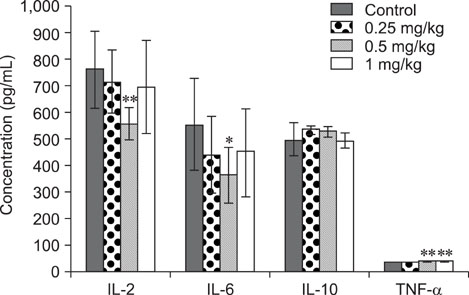
- The objective of this study was to explore the immunomodulatory effects of betulinic acid (BA) extracted from the bark of white birch on mice. Female mice were orally administered BA for 14 days in doses of 0, 0.25, 0.5, and 1 mg/kg body weight. We found that BA significantly enhanced the thymus and spleen indices, and stimulated lymphocyte proliferation induced by Concanavalin A and lipopolysaccharide as shown by MTT assay. Flow cytometry revealed that BA increased the percentage of CD4+ cells in thymus as well as the percentage of CD19+ and the ratios of CD4+/CD8+ in spleen. BA increased the number of plaque-forming cell and macrophage phagocytic activity as indicated by a neutral red dye uptake assay, and the peritoneal macrophages levels of TNF-alpha were also increased. In contrast, serum levels of IgG and IgM and serum concentrations of IL-2 and IL-6 were significantly decreased in BA-treated mice compared to the control as assayed by haemagglutination tests and ELISA, respectively. Taken together, these results suggest that BA enhances mouse cellular immunity, humoral immunity, and activity of macrophages. Thus, BA is a potential immune stimulator and may strengthen the immune response of its host.
MeSH Terms
-
Adaptive Immunity/*drug effects
Animals
Betula/*chemistry
Cell Proliferation/drug effects
Cytokines/blood
Female
Immunity, Innate/*drug effects
Immunologic Factors/*pharmacology
Macrophages/drug effects
Mice
Phagocytosis/drug effects
Random Allocation
Spleen/cytology
Thymus Gland/cytology
Triterpenes/*pharmacology
Figure
Cited by 1 articles
-
Betulinic acid prevents alcohol-induced liver damage by improving the antioxidant system in mice
Jine Yi, Wei Xia, Jianping Wu, Liyun Yuan, Jing Wu, Di Tu, Jun Fang, Zhuliang Tan
J Vet Sci. 2014;15(1):141-148. doi: 10.4142/jvs.2014.15.1.141.
Reference
-
1. Adler FL. Studies on mouse antibodies. II. Mercaptoethanol-sensitive 7 S antibodies in mouse antisera to protein antigens. J Immunol. 1965. 95:39–47.2. Alakurtti S, Mäkelä T, Koskimies S, Yli-Kauhaluoma J. Pharmacological properties of the ubiquitous natural product betulin. Eur J Pharm Sci. 2006. 29:1–13.
Article3. Al-Daccak R, Mooney N, Charron D. MHC class II signaling in antigen-presenting cells. Curr Opin Immunol. 2004. 16:108–113.
Article4. Amat N, Upur H, Ablimit A, Matsidik A, Yusup A, Kijjoa A. Immunomodulatory effects of Abnormal Savda Munsiq, a traditional Uighur medicine, on the combined stress mice. J Ethnopharmacol. 2009. 122:42–47.
Article5. Bernard P, Scior T, Didier B, Hibert M, Berthon JY. Ethnopharmacology and bioinformatic combination for leads discovery: application to phospholipase A2 inhibitors. Phytochemistry. 2001. 58:865–874.
Article6. Bringmann G, Saeb W, Assi LA, François G, Sankara Narayanan AS, Peters K, Peters EM. Betulinic acid: isolation from Triphyophyllum peltatum and Ancistrocladus heyneanus, antimalarial activity, and crystal structure of the benzyl ester. Planta Med. 1997. 63:255–257.
Article7. Chang CW, Wu TS, Hsieh YS, Kuo SC, Chao PD. Terpenoids of Syzygium formosanum. J Nat Prod. 1999. 62:327–328.8. Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: The alternative approaches. Annu Rev Immunol. 1997. 15:297–322.
Article9. Dhur A, Galan P, Preziosi P, Hercberg S. Lymphocyte subpopulations in the thymus, lymph nodes and spleen of iron-deficient and rehabilitated mice. J Nutr. 1991. 121:1418–1424.
Article10. Enwerem NM, Okogun JI, Wambebe CO, Okorie DA, Akah PA. Anthelmintic activity of the stem bark extracts of Berlina grandiflora and one of its active principles, Betulinic acid. Phytomedicine. 2001. 8:112–114.
Article11. Esper RJ, Nordaby RA, Vilariño JO, Paragano A, Cacharrón JL, Machado RA. Endothelial dysfunction: a comprehensive appraisal. Cardiovasc Diabetol. 2006. 5:4.12. Flekhter OB, Nigmatullina LR, Baltina LA, Karachurina LT, Galin FZ, Zarudii FS, Tolstikov GA, Boreko EI, Pavlova NI, Nikolaeva SN, Savinova OV. Synthesis of betulinic acid from betulin extract and study of the antiviral and antiulcer activity of some related terpenoids. Pharm Chem J. 2002. 36:484–487.13. Fujioka T, Kashiwada Y, Kilkuskie RE, Cosentino LM, Ballas LM, Jiang JB, Janzen WP, Chen IS, Lee KH. Anti-AIDS agents, 11. Betulinic acid and platanic acid as anti-HIV principles from Syzigium claviflorum, and the anti-HIV activity of structurally related triterpenoids. J Nat Prod. 1994. 57:243–247.
Article14. Gautam M, Saha S, Bani S, Kaul A, Mishra S, Patil D, Satti NK, Suri KA, Gairola S, Suresh K, Jadhav S, Qazi GN, Patwardhan B. Immunomodulatory activity of Asparagus racemosus on systemic Th1/Th2 immunity: implications for immunoadjuvant potential. J Ethnopharmacol. 2009. 121:241–247.
Article15. Higa M, Noha N, Yokaryo H, Ogihara K, Yogi S. Three new naphthoquinone derivatives from Diospyros maritima Blume. Chem Pharm Bull (Tokyo). 2002. 50:590–593.
Article16. Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM. The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J. 2001. 15:43–58.
Article17. Kamimura D, Ishihara K, Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 2003. 149:1–38.
Article18. Kovalenko LP, Balakshin VV, Presnova GA, Chistyakov AN, Shipaeva EV, Alekseeva SV, Durnev AD. Immunotoxicity and allergenic properties of betulin-containing birch bark dry extract. Pharm Chem J. 2007. 41:17–19.
Article19. Ma MS, Bae IY, Lee HG, Yang CB. Purification and identification of angiotensin I-converting enzyme inhibitory peptide from buckwheat (Fagopyrum esculentum Moench). Food Chem. 2006. 96:36–42.
Article20. Meisel H. Food-derived bioactive proteins and peptides as potential components of nutraceuticals. Curr Pharm Des. 2007. 13:873–874.
Article21. O'Garra A, Murphy K. Role of cytokines in determining T-lymphocyte function. Curr Opin Immunol. 1994. 6:458–466.22. Pisha E, Chai H, Lee IS, Chagwedera TE, Farnsworth NR, Cordell GA, Beecher CW, Fong HH, Kinghorn AD, Brown DM, Wani MC, Wall ME, Hieken TJ, Das Gupta TK, Pezzuto JM. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat Med. 1995. 1:1046–1051.
Article23. Pokrovskii AG, Shintyapina AB, Pronkina NV, Kozhevnikov VS, Plyasunova OA, Shul'ts EE, Tolstikov GA. Activation of apoptosis by derivatives of betulinic acid in human tumor cells in vitro. Dokl Biochem Biophys. 2006. 407:94–97.
Article24. Recio MC, Giner RM, Máñez S, Gueho J, Julien HR, Hostettmann K, Ríos JL. Investigations on the steroidal anti-inflammatory activity of triterpenoids from Diospyros leucomelas. Planta Med. 1995. 61:9–12.
Article25. Schühly W, Heilmann J, Calis I, Sticher O. New triterpenoids with antibacterial activity from Zizyphus joazeiro. Planta Med. 1999. 65:740–743.
Article26. Scheller J, Ohnesorge N, Rose-John S. Interleukin-6 trans-signalling in chronic inflammation and cancer. Scand J Immunol. 2006. 63:321–329.
Article27. Takahashi M, Moriguchi S, Yoshikawa M, Sasaki R. Isolation and characterization of oryzatensin: A novel bioactive peptide with ileum-contracting and immunomodulating activities derived from rice albumin. Biochem Mol Biol Int. 1994. 33:1151–1158.28. Yamauchi F, Suetsuna K. Immunological effects of dietary peptide derived from soybean protein. J Nutr Biochem. 1993. 4:450–457.
Article29. Yang R, Zhang Z, Pei X, Han X, Wang J, Wang L, Long Z, Shen X, Li Y. Immunomodulatory effects of marine oligopeptide preparation from Chum Salmon (Oncorhynchus keta) in mice. Food Chem. 2009. 113:464–470.
Article30. Yun Y, Han S, Park E, Yim D, Lee S, Lee CK, Cho K, Kim K. Immunomodulatory activity of betulinic acid by producing pro-inflammatory cytokines and activation of macrophages. Arch Pharm Res. 2003. 26:1087–1095.
Article31. Zdzisińska B, Rzeski W, Paduch R, Szuster-Ciesielska A, Kaczor J, Wejksza K, Kandefer-Szerszeń M. Differential effect of betulin and betulinic acid on cytokine production in human whole blood cell cultures. Pol J Pharmacol. 2003. 55:235–238.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of the Sanjoin on the Rat Brain: Focused on Serotonin, Sleeping Time, Sleep EEG and Autonomic Activity
- Anti-Influenza Activity of Betulinic Acid from Zizyphus jujuba on Influenza A/PR/8 Virus
- Inhibition of Tumor Growth and Angiogenesis by KJ3, Betulinic Acid, and Fumagillin in Mouse Neuroblastoma
- Effect of Betulinic Acid on MUC5AC and MUC5B Expression in Airway Epithelial Cells
- Betulinic acid prevents alcohol-induced liver damage by improving the antioxidant system in mice