J Vet Sci.
2011 Sep;12(3):295-297. 10.4142/jvs.2011.12.3.295.
Development of a novel diagnostic test for detection of bovine viral diarrhea persistently infected animals using hair
- Affiliations
-
- 1Veterinary Diagnostic Laboratory, College of Veterinary Medicine, University of Illinois, Urbana, IL 61802, USA. ksingh08@illinois.edu
- 2Veterinary Teaching Hospital, College of Veterinary Medicine, University of Illinois, Urbana, IL 61802, USA.
- 3Department of Veterinary Science, Wyoming State Veterinary Laboratory, Laramie, WY 82070, USA.
- KMID: 1067404
- DOI: http://doi.org/10.4142/jvs.2011.12.3.295
Abstract
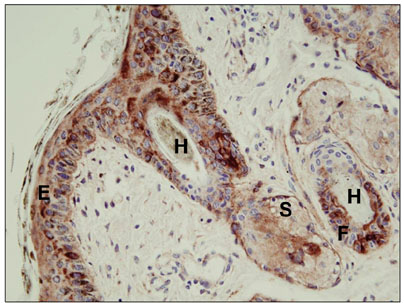
- The purpose of this study was to determine whether manually plucked hairs might serve as an alternative sample for a quantitative real time polymerase chain reaction (qRT-PCR) testing. Twenty three, 1~3 week old, non-bovine viral diarrhea virus (BVDV) vaccinated calves, found to be positive for BVDV by immunohistochemical staining, were selected and hairs were manually plucked from the ear. qRT-PCR was performed on samples consisting of more than 30 hairs (30~100) and whole blood. All 23 animals were positive for the virus by qRT-PCR performed on the whole blood and when samples of more than 30 hairs were assayed. Additionally, qRT-PCR was performed on groups of 10 and 20 hairs harvested from 7 out of 23 immunohistochemical staining-positive calves. When groups of 20 and 10 hairs were tested, 6 and 4 animals, respectively, were positive for the virus.
MeSH Terms
-
Animals
Antibodies, Viral/analysis/diagnostic use
Bovine Virus Diarrhea-Mucosal Disease/blood/*diagnosis/virology
Cattle
Diarrhea Virus 1, Bovine Viral/genetics/*isolation & purification
Diarrhea Virus 2, Bovine Viral/genetics/*isolation & purification
Hair/virology
RNA, Viral/chemistry/genetics
Real-Time Polymerase Chain Reaction/methods/*veterinary
Figure
Reference
-
1. Bhudevi B, Weinstock D. Fluorogenic RT-PCR assay (TaqMan) for detection and classification of bovine viral diarrhea virus. Vet Microbiol. 2001. 83:1–10.
Article2. Cornish TE, van Olphen AL, Cavender JL, Edwards JM, Jaeger PT, Vieyra LL, Woodard LF, Miller DR, O'Toole D. Comparison of ear notch immunohistochemistry, ear notch antigen-capture ELISA, and buffy coat virus isolation for detection of calves persistently infected with bovine viral diarrhea virus. J Vet Diagn Invest. 2005. 17:110–117.
Article3. Driskell EA, Ridpath JF. A survey of bovine viral diarrhea virus testing in diagnostic laboratories in the United States from 2004 to 2005. J Vet Diagn Invest. 2006. 18:600–605.
Article4. Reichel MP, Hill FI, Voges H. Does control of bovine viral diarrhoea infection make economic sense? N Z Vet J. 2008. 56:60–66.
Article5. Ridpath JF, Fulton RW. Knowledge gaps impacting the development of bovine viral diarrhea virus control programs in the United States. J Am Vet Med Assoc. 2009. 235:1171–1179.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tissue distribution of bovine viral diarrhea virus antigens in persistently infected cattle
- Genetic characterization of bovine viral diarrhea virus strains in Beijing, China and innate immune responses of peripheral blood mononuclear cells in persistently infected dairy cattle
- Prevalence of bovine viral diarrhea virus from Korean native cattle farms in Jeju
- Genetic Typing of Bovine Viral Diarrhea Viruses (BVDV) Circulating in Korea
- Klebsiella pneumoniae infection secondary to bovine viral diarrhea in two prematurely born calves