Yonsei Med J.
2011 Nov;52(6):953-960. 10.3349/ymj.2011.52.6.953.
The Association of SERPINE2 Gene with COPD in a Chinese Han Population
- Affiliations
-
- 1Department of Respiratory, Qilu Hospital of Shandong University, Shandong, China. Xiaowei4226@163.com
- 2Department of Pulmonary Medicine, Yuebei People's Hospital, Shaoguan, China.
- 3Department of Pharmacy, Shandong Provincial Hospital, Shandong, China.
- 4Department of medical genetics, School of Medicine, Shandong University, Shandong, China.
- KMID: 1058815
- DOI: http://doi.org/10.3349/ymj.2011.52.6.953
Abstract
- PURPOSE
Polymorphisms of several candidate genes have been studied and associated with the development of chronic obstructive pulmonary disease (COPD). One such candidate is the SERPINE2 (Serpin peptidase inhibitor, clade E member 2) gene.
MATERIALS AND METHODS
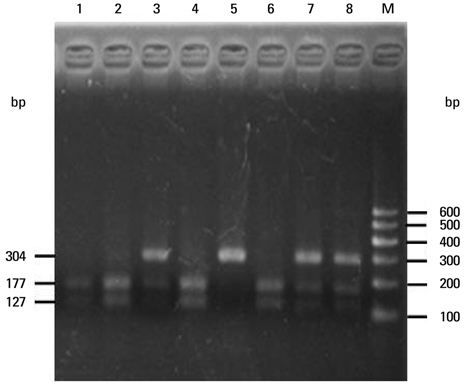
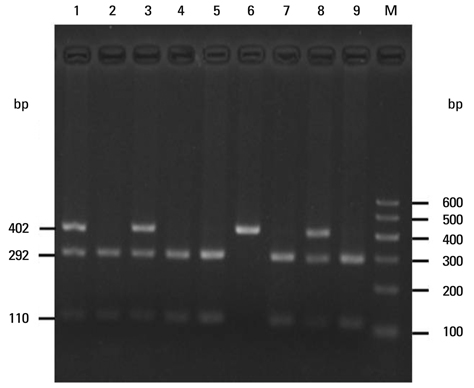
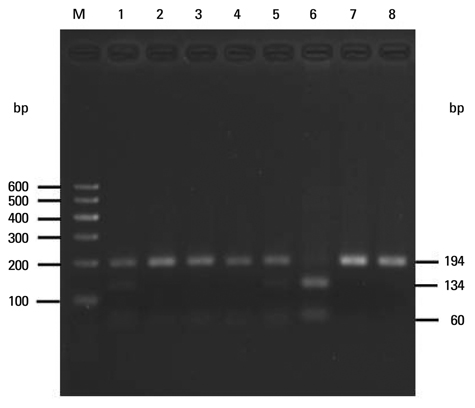
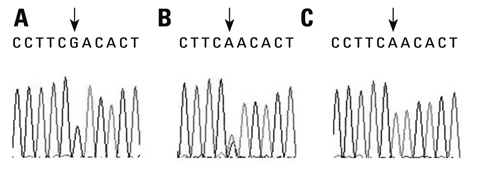
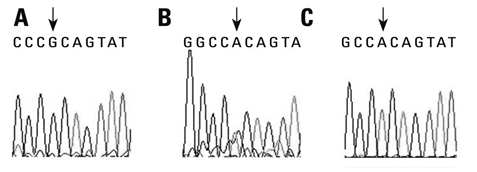
To assess whether the SERPINE2 gene is associated with COPD in a Chinese Han population. Samples were collected from a Chinese Han population and analyzed for the association of single nucleotide polymor phisms (SNPs) or haplotypes of SERPINE2 gene with COPD in a case-control study. Three SNPs including rs840088 G/A in intron 1, rs1438831 A/G in 5' upstream sequence and rs3795879 G/A in intron 3 were detected using the polymerase chain reaction (PCR)-based restriction fragment length polymorphism technique in 409 COPD subjects and 411 controls. Genotyping of the SREPINE2 polymorphisms at positions rs840088, rs1438831and rs3795879 was performed.
RESULTS
We found that none of the rs840088G/A, rs1438831G/A and rs3795879 G/A polymorphisms were associated with the disease. The p-values were 0.630, 0.208 and 0.398 respectively.
CONCLUSION
Our data suggested that there was no significant association between SERPINE2 polymorphism and COPD susceptibility in the Chinese Han population.
MeSH Terms
-
Asian Continental Ancestry Group
Case-Control Studies
Female
Genetic Predisposition to Disease/genetics
Genotype
Haplotypes/genetics
Humans
Male
Middle Aged
Polymorphism, Restriction Fragment Length/genetics
Polymorphism, Single Nucleotide/genetics
Pulmonary Disease, Chronic Obstructive/*genetics
Serpin E2/*genetics
Figure
Reference
-
1. Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001. 163:1256–1276.
Article2. World Health Report. Geneva: World Health Organization;Available from URL: http://www.who.int/research/en/.3. Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006. 27:397–412.
Article4. Lieberman J, Winter B, Sastre A. Alpha 1-antitrypsin Pi-types in 965 COPD patients. Chest. 1986. 89:370–373.5. Celli BR, Halbert RJ, Nordyke RJ, Schau B. Airway obstruction in never smokers: results from the Third National Health and Nutrition Examination Survey. Am J Med. 2005. 118:1364–1372.
Article6. Behrendt CE. Mild and moderate-to-severe COPD in nonsmokers: distinct demographic profiles. Chest. 2005. 128:1239–1244.7. Sandford AJ, Silverman EK. Chronic obstructive pulmonary disease. 1: susceptibility factors for COPD the genotype-environment interaction. Thorax. 2002. 57:736–741.
Article8. Sandford AJ, Joos L, Paré PD. Genetic risk factors for chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2002. 8:87–94.
Article9. Molfino NA. Current thinking on genetics of chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2007. 13:107–113.
Article10. Eddahibi S, Chaouat A, Morrell N, Fadel E, Fuhrman C, Bugnet AS, et al. Polymorphism of the serotonin transporter gene and pulmonary hypertension in chronic obstructive pulmonary disease. Circulation. 2003. 108:1839–1844.
Article11. Yildiz P, Oflaz H, Cine N, Erginel-Unaltuna N, Erzengin F, Yilmaz V. Gene polymorphisms of endothelial nitric oxide synthase enzyme associated with pulmonary hypertension in patients with COPD. Respir Med. 2003. 97:1282–1288.
Article12. Castaldi PJ, Hersh CP, Reilly JJ, Silverman EK. Genetic associations with hypoxemia and pulmonary arterial pressure in COPD. Chest. 2009. 135:737–744.
Article13. Demeo DL, Mariani TJ, Lange C, Srisuma S, Litonjua AA, Celedon JC, et al. The SERPINE2 gene is associated with chronic obstructive pulmonary disease. Am J Hum Genet. 2006. 78:253–264.
Article14. Zhu G, Warren L, Aponte J, Gulsvik A, Bakke P, Anderson WH, et al. The SERPINE2 gene is associated with chronic obstructive pulmonary disease in two large populations. Am J Respir Crit Care Med. 2007. 176:167–173.
Article15. Chappell S, Daly L, Morgan K, Baranes TG, Roca J, Rabinovich R, et al. The SERPINE2 gene and chronic obstructive pulmonary disease. Am J Hum Genet. 2006. 79:184–186. author reply 186-7.16. American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995. 152:S77–S121.17. Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988. 16:1215.
Article18. Smith OO, Helms PJ. Genetic/environmental determinants of adult chronic obstructive pulmonary disease and possible links with childhood wheezing. Paediatr Respir Rev. 2001. 2:178–183.
Article19. Carter RE, Cerosaletti KM, Burkin DJ, Fournier RE, Jones C, Greenberg BD, et al. The gene for the serpin thrombin inhibitor (PI7), protease nexin I, is located on human chromosome 2q33-q35 and on syntenic regions in the mouse and sheep genomes. Genomics. 1995. 27:196–199.
Article20. Rossignol P, Ho-Tin-Noé B, Vranckx R, Bouton MC, Meilhac O, Lijnen HR, et al. Protease nexin-1 inhibits plasminogen activation-induced apoptosis of adherent cells. J Biol Chem. 2004. 279:10346–10356.
Article21. Mbebi C, Rohn T, Doyennette MA, Chevessier F, Jandrot-Perrus M, Hantaï D, et al. Thrombin receptor induction by injury-related factors in human skeletal muscle cells. Exp Cell Res. 2001. 263:77–87.
Article22. Lohmueller KE, Pearce CL, Pike M, Lander ES, Hirschhorn JN. Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet. 2003. 33:177–182.
Article23. Hersh CP, Demeo DL, Lange C, Litonjua AA, Reilly JJ, Kwiatkowski D, et al. Attempted replication of reported chronic obstructive pulmonary disease candidate gene associations. Am J Respir Cell Mol Biol. 2005. 33:71–78.
Article24. Silverman EK, Palmer LJ. Case-control association studies for the genetics of complex respiratory diseases. Am J Respir Cell Mol Biol. 2000. 22:645–648.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- SERPINE2 Polymorphisms and Chronic Obstructive Pulmonary Disease
- Association between Histone Deacetylase 9 Gene Polymorphism and Stroke in Chinese Han Population
- -1438A/G Polymorphism of the 5-HT2A Receptor Gene in Korean and Han Chinese Patients with Schizophrenia
- The Gene Polymorphism of VMAT2 Is Associated with Risk of Schizophrenia in Male Han Chinese
- Association between the Human Surfactant Protein-A(SP-A) Gene Locus and Chronic Obstructive Pulmonary Disease in Korean Population