Yonsei Med J.
2011 Nov;52(6):939-947. 10.3349/ymj.2011.52.6.939.
Parameters Measuring Beta-Cell Function Are Only Valuable in Diabetic Subjects with Low Body Mass Index, High Blood Glucose Level, or Long-Standing Diabetes
- Affiliations
-
- 1Department of Internal Medicine, Myongji Hospital, Kwandong University College of Medicine, Goyang, Korea. khj121210@paran.com
- KMID: 1058813
- DOI: http://doi.org/10.3349/ymj.2011.52.6.939
Abstract
- PURPOSE
The aim of this study was to identify the most precise and clinically practicable parameters that predict future oral hypoglycemic agent (OHA) failure in patients with type 2 diabetes, and to determine whether these parameters are valuable in various subgroups.
MATERIALS AND METHODS
We took fasting blood samples from 231 patients for laboratory data and standard breakfast tests for evaluation of pancreatic beta-cell function. Hemoglobin A1c (HbA1c) levels were tested, and we collected data related to hypoglycemic medications one year from the start date of the study.
RESULTS
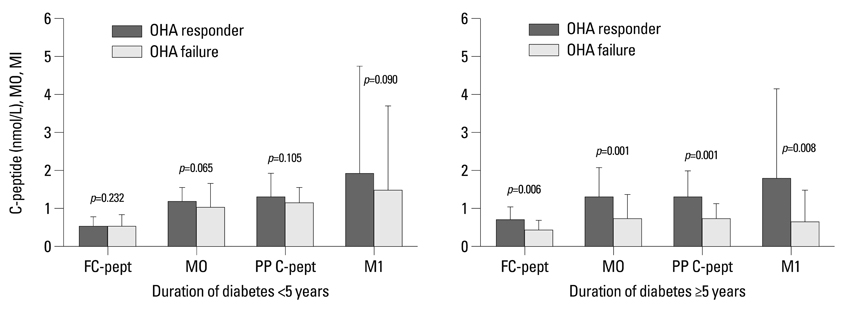
Fasting C-peptide, postprandial insulin and C-peptide, the difference between fasting and postprandial insulin, fasting beta-cell responsiveness (M0), postprandial beta-cell responsiveness (M1), and homeostasis model assessment-beta (HOMA-B) levels were significantly higher in those with OHA response than in those with OHA failure. The area under the curve (AUC) of the receiver operating characteristic (ROC) measured with postprandial C-peptide to predict future OHA failure was 0.720, and the predictive power for future OHA failure was the highest of the variable parameters. Fasting and postprandial C-peptide, M0, and M1 levels were the only differences between those with OHA response and those with OHA failure among diabetic subjects with low body mass index, high blood glucose level, or long-standing diabetes.
CONCLUSION
In conclusion, postprandial C-peptide was most useful in predicting future OHA failure in type 2 diabetic subjects. However, these parameters measuring beta-cell function are only valuable in diabetic subjects with low body mass index, high blood glucose level, or long-standing diabetes.
MeSH Terms
-
Administration, Oral
Adolescent
Aged
Blood Glucose/analysis
Body Mass Index
C-Peptide/blood
Child
Child, Preschool
Diabetes Mellitus, Type 2/*blood/drug therapy/*metabolism/physiopathology
Fasting/blood
Female
Humans
Hypoglycemic Agents/administration & dosage/therapeutic use
Insulin/blood
Insulin-Secreting Cells/*metabolism/*physiology
Male
Middle Aged
Postprandial Period
Figure
Reference
-
1. Seltzer HS, Harris VL. Exhaustion of insulogenic reserve in maturity-onset diabetic patients during prolonged and continuous hyperglycemic stress. Diabetes. 1964. 13:6–13.
Article2. Groop LC, Pelkonen R, Koskimies S, Bottazzo GF, Doniach D. Secondary failure to treatment with oral antidiabetic agents in non-insulin-dependent diabetes. Diabetes Care. 1986. 9:129–133.
Article3. U.K. Prospective Diabetes Study Group. U.K. prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. Diabetes. 1995. 44:1249–1258.4. Festa A, Williams K, D'Agostino R Jr, Wagenknecht LE, Haffner SM. The natural course of beta-cell function in nondiabetic and diabetic individuals: the Insulin Resistance Atherosclerosis Study. Diabetes. 2006. 55:1114–1120.
Article5. Hutton JC. Insulin secretory granule biogenesis and the proinsulin-processing endopeptidases. Diabetologia. 1994. 37:Suppl 2. S48–S56.
Article6. Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009. 32:193–203.
Article7. Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990. 51:241–247.
Article8. Larsen PR, Kronenberg HM, Melmed S, Polonsky KS. Williams textbook of endocrinology. 2003. 10th ed. Philadelphia: SAUNDERS.9. Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000. 85:2402–2410.
Article10. Albareda M, Rodriguez-Espinosa J, Murugo M, de Leiva A, Corcoy R. Assessment of insulin sensitivity and beta-cell function from measurements in the fasting state and during an oral glucose tolerance test. Diabetologia. 2000. 43:1507–1511.
Article11. Hovorka R, Chassin L, Luzio SD, Playle R, Owens DR. Pancreatic beta-cell responsiveness during meal tolerance test: model assessment in normal subjects and subjects with newly diagnosed noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1998. 83:744–750.
Article12. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003. 289:2560–2572.
Article13. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001. 285:2486–2497.14. American Diabetes Association. Standards of medical care in diabetes--2006. Diabetes Care. 2006. 29:Suppl 1. S4–S42.15. Ryan TJ, Antman EM, Brooks NH, Califf RM, Hillis LD, Hiratzka LF, et al. 1999 update: ACC/AHA Guidelines for the Management of Patients With Acute Myocardial Infarction: Executive Summary and Recommendations: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Acute Myocardial Infarction). Circulation. 1999. 100:1016–1030.
Article16. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979. 237:E214–E223.
Article17. Pacini G, Mari A. Methods for clinical assessment of insulin sensitivity and beta-cell function. Best Pract Res Clin Endocrinol Metab. 2003. 17:305–322.
Article18. Scheen AJ, Castillo MJ, Lefébvre PJ. Assessment of residual insulin secretion in diabetic patients using the intravenous glucagon stimulatory test: methodological aspects and clinical applications. Diabetes Metab. 1996. 22:397–406.19. Koskinen PJ, Viikari JS, Irjala KM. Glucagon-stimulated and postprandial plasma C-peptide values as measures of insulin secretory capacity. Diabetes Care. 1988. 11:318–322.
Article20. Hovorka R, Jones RH. How to measure insulin secretion. Diabetes Metab Rev. 1994. 10:91–117.
Article21. Hovorka R, Chassin L, Luzio SD, Playle R, Owens DR. Pancreatic beta-cell responsiveness during meal tolerance test: model assessment in normal subjects and subjects with newly diagnosed noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1998. 83:744–750.
Article22. Reaven GM, Chen YD, Hollenbeck CB, Sheu WH, Ostrega D, Polonsky KS. Plasma insulin, C-peptide, and proinsulin concentrations in obese and nonobese individuals with varying degrees of glucose tolerance. J Clin Endocrinol Metab. 1993. 76:44–48.
Article23. Yudkin JS. Circulating proinsulin-like molecules. J Diabetes Complications. 1993. 7:113–123.
Article24. Manzanares JM, Conget I, Gonzalez-Clemente JM, Vidal J, Rodríguez-Villar C, Rojas I, et al. [Insulin treatment in diabetes mellitus type II: the usefulness of the breakfast test]. Med Clin (Barc). 1995. 104:761–764.25. Pontiroli AE, Calderara A, Maffi P, Bonisolli L, Carenini A, Piatti PM, et al. Secondary failure to oral hypoglycaemic agents in non-obese patients with non-insulin-dependent diabetes is related to reduced insulin release. Diabete Metab. 1989. 15:79–84.26. Peiris AN, Mueller RA, Smith GA, Struve MF, Kissebah AH. Splanchnic insulin metabolism in obesity. Influence of body fat distribution. J Clin Invest. 1986. 78:1648–1657.
Article27. Gjessing HJ, Reinholdt B, Faber OK, Pedersen O. The effect of acute hyperglycemia on the plasma C-peptide response to intravenous glucagon or to a mixed meal in insulin-dependent diabetes mellitus. Acta Endocrinol (Copenh). 1991. 124:556–562.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Normal Glucose Tolerance with a High 1-Hour Postload Plasma Glucose Level Exhibits Decreased beta-Cell Function Similar to Impaired Glucose Tolerance
- Beta-Cell Function and Nutrient Intake
- Serum Insulin, Proinsulin and Proinsulin/Insulin Ratio in Type 2 Diabetic Patients: As an Index of beta-Cell Function or Insulin Resistance
- Insulin Sensitivity and Insulin Secretion Determined by Homeostasis Model Assessment and Future Risk of Diabetes Mellitus in Korean Men
- Fasting Serum Insulin Levels in Relation to Age and Body Mass Index and Serum Glucose Level in Healthy Subjects in Korea