Korean J Radiol.
2011 Feb;12(1):34-43. 10.3348/kjr.2011.12.1.34.
Computer-Aided Evaluation of Breast MRI for the Residual Tumor Extent and Response Monitoring in Breast Cancer Patients Receiving Neoadjuvant Chemotherapy
- Affiliations
-
- 1Department of Radiology and Clinical Research Institute, Seoul National University Hospital and the Institute of Radiation Medicine, Seoul National University Medical Research Center, Seoul 110-744, Korea. nariya@radiol.snu.ac.kr
- 2Department of Radiology, Seoul National University Bundang Hospital, Gyeonggi-do 463-707, Korea.
- 3Department of Radiology, Hanyang University College of Medicine, Hanyang University Hospital, Seoul 133-792, Korea.
- 4Department of Radiology, School of Medicine, Ewha Womans University, Seoul 158-710, Korea.
- KMID: 991680
- DOI: http://doi.org/10.3348/kjr.2011.12.1.34
Abstract
OBJECTIVE
To evaluate the accuracy of a computer-aided evaluation program (CAE) of breast MRI for the assessment of residual tumor extent and response monitoring in breast cancer patients receiving neoadjuvant chemotherapy.
MATERIALS AND METHODS
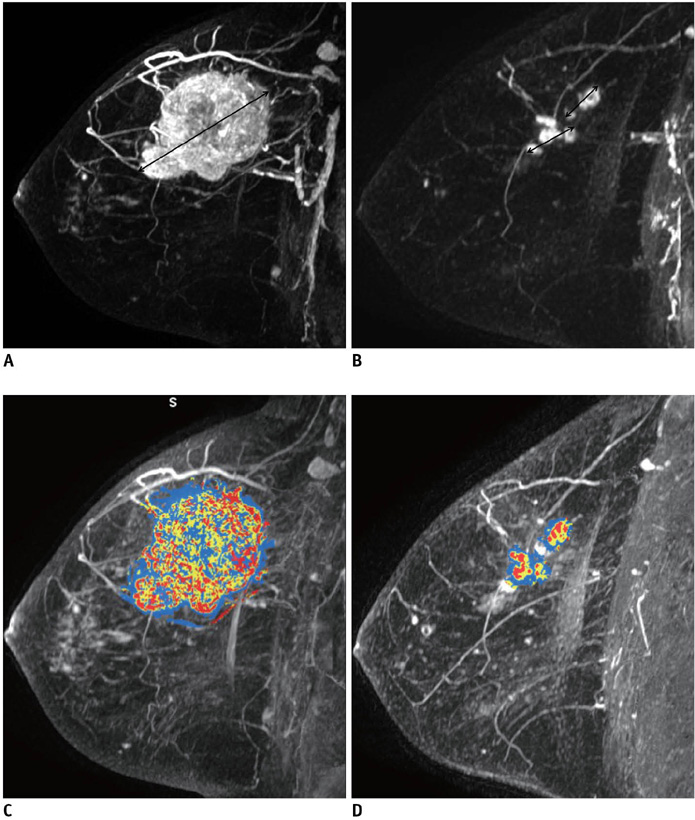
Fifty-seven patients with breast cancers who underwent neoadjuvant chemotherapy before surgery and dynamic contrast enhanced MRI before and after chemotherapy were included as part of this study. For the assessment of residual tumor extent after completion of chemotherapy, the mean tumor diameters measured by radiologists and CAE were compared to those on histopathology using a paired student t-test. Moreover, the agreement between unidimensional (1D) measurement by radiologist and histopathological size or 1D measurement by CAE and histopathological size was assessed using the Bland-Altman method. For chemotherapy monitoring, we evaluated tumor response through the change in the 1D diameter by a radiologist and CAE and three-dimensional (3D) volumetric change by CAE based on Response Evaluation Criteria in Solid Tumors (RECIST). Agreement between the 1D response by the radiologist versus the 1D response by CAE as well as by the 3D response by CAE were evaluated using weighted kappa (k) statistics.
RESULTS
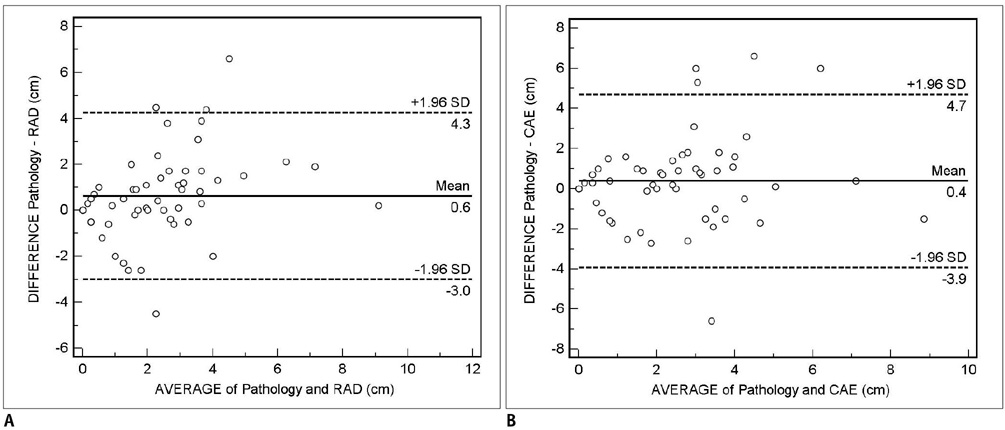
For the assessment of residual tumor extent after chemotherapy, the mean tumor diameter measured by radiologists (2.0 +/- 1.7 cm) was significantly smaller than the mean histological diameter (2.6 +/- 2.3 cm) (p = 0.01), whereas, no significant difference was found between the CAE measurements (mean = 2.2 +/- 2.0 cm) and histological diameter (p = 0.19). The mean difference between the 1D measurement by the radiologist and histopathology was 0.6 cm (95% confidence interval: -3.0, 4.3), whereas the difference between CAE and histopathology was 0.4 cm (95% confidence interval: -3.9, 4.7). For the monitoring of response to chemotherapy, the 1D measurement by the radiologist and CAE showed a fair agreement (k = 0.358), while the 1D measurement by the radiologist and 3D measurement by CAE showed poor agreement (k = 0.106).
CONCLUSION
CAE for breast MRI is sufficiently accurate for the assessment of residual tumor extent in breast cancer patients receiving neoadjuvant chemotherapy. However, for the assessment of response to chemotherapy, the assessment by the radiologist and CAE showed a fair to poor agreement.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Comparison of the Diagnostic Performance of Response Evaluation Criteria in Solid Tumor 1.0 with Response Evaluation Criteria in Solid Tumor 1.1 on MRI in Advanced Breast Cancer Response Evaluation to Neoadjuvant Chemotherapy
Su Kyung Jeh, Sung Hun Kim, Bong Joo Kang
Korean J Radiol. 2013;14(1):13-20. doi: 10.3348/kjr.2013.14.1.13.Early Prediction of Response to Neoadjuvant Chemotherapy Using Dynamic Contrast-Enhanced MRI and Ultrasound in Breast Cancer
Yunju Kim, Sung Hun Kim, Byung Joo Song, Bong Joo Kang, Kwang-il Yim, Ahwon Lee, Yoonho Nam
Korean J Radiol. 2018;19(4):682-691. doi: 10.3348/kjr.2018.19.4.682.
Reference
-
1. Esteva FJ, Hortobagyi GN. Locally advanced breast cancer. Hematol Oncol Clin North Am. 1999. 13:457–472.2. Kaufmann M, von Minckwitz G, Smith R, Valero V, Gianni L, Eiermann W, et al. International expert panel on the use of primary (preoperative) systemic treatment of operable breast cancer: review and recommendations. J Clin Oncol. 2003. 21:2600–2608.3. Estévez LG, Gradishar WJ. Evidence-based use of neoadjuvant taxane in operable and inoperable breast cancer. Clin Cancer Res. 2004. 10:3249–3261.4. Shimizu C, Ando M, Kouno T, Katsumata N, Fujiwara Y. Current trends and controversies over pre-operative chemotherapy for women with operable breast cancer. Jpn J Clin Oncol. 2007. 37:1–8.5. Buchholz TA, Lehman CD, Harris JR, Pockaj BA, Khouri N, Hylton NF, et al. Statement of the science concerning locoregional treatments after preoperative chemotherapy for breast cancer: a National Cancer Institute conference. J Clin Oncol. 2008. 26:791–797.6. Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998. 16:2672–2685.7. Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999. 17:460–469.8. Bonadonna G, Valagussa P, Brambilla C, Ferrari L, Moliterni A, Terenziani M, et al. Primary chemotherapy in operable breast cancer: eight-year experience at the Milan Cancer Institute. J Clin Oncol. 1998. 16:93–100.9. Buchholz TA, Hill BS, Tucker SL, Frye DK, Kuerer HM, Buzdar AU, et al. Factors predictive of outcome in patients with breast cancer refractory to neoadjuvant chemotherapy. Cancer J. 2001. 7:413–420.10. Lee SH, Cho N, Kim SJ, Cha JH, Cho KS, Ko ES, et al. Correlation between high resolution dynamic MR features and prognostic factors in breast cancer. Korean J Radiol. 2008. 9:10–18.11. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000. 92:205–216.12. Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: a review of validation studies on tumour assessment. Eur J Cancer. 2006. 42:1031–1039.13. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009. 45:228–247.14. Balu-Maestro C, Chapellier C, Bleuse A, Chanalet I, Chauvel C, Largillier R. Imaging in evaluation of response to neoadjuvant breast cancer treatment benefits of MRI. Breast Cancer Res Treat. 2002. 72:145–152.15. Abraham DC, Jones RC, Jones SE, Cheek JH, Peters GN, Knox SM, et al. Evaluation of neoadjuvant chemotherapeutic response of locally advanced breast cancer by magnetic resonance imaging. Cancer. 1996. 78:91–100.16. Drew PJ, Kerin MJ, Mahapatra T, Malone C, Monson JR, Turnbull LW, et al. Evaluation of response to neoadjuvant chemoradiotherapy for locally advanced breast cancer with dynamic contrast-enhanced MRI of the breast. Eur J Surg Oncol. 2001. 27:617–620.17. Gilles R, Guinebretière JM, Toussaint C, Spielman M, Rietjens M, Petit JY, et al. Locally advanced breast cancer: contrast-enhanced subtraction MR imaging of response to preoperative chemotherapy. Radiology. 1994. 191:633–638.18. Partridge SC, Gibbs JE, Lu Y, Esserman LJ, Sudilovsky D, Hylton NM. Accuracy of MR imaging for revealing residual breast cancer in patients who have undergone neoadjuvant chemotherapy. AJR Am J Roentgenol. 2002. 179:1193–1999.19. Weatherall PT, Evans GF, Metzger GJ, Saborrian MH, Leitch AM. MRI vs. histologic measurement of breast cancer following chemotherapy: comparison with x-ray mammography and palpation. J Magn Reson Imaging. 2001. 13:868–875.20. Rosen EL, Blackwell KL, Baker JA, Soo MS, Bentley RC, Yu D, et al. Accuracy of MRI in the detection of residual breast cancer after neoadjuvant chemotherapy. AJR Am J Roentgenol. 2003. 181:1275–1282.21. Rieber A, Zeitler H, Rosenthal H, Görich J, Kreienberg R, Brambs HJ, et al. MRI of breast cancer: influence of chemotherapy on sensitivity. Br J Radiol. 1997. 70:452–458.22. Wasser K, Sinn HP, Fink C, Klein SK, Junkermann H, Lüdemann HP, et al. Accuracy of tumor size measurement in breast cancer using MRI is influenced by histological regression induced by neoadjuvant chemotherapy. Eur Radiol. 2003. 13:1213–1223.23. Demartini WB, Lehman CD, Peacock S, Russell MT. Computer-aided detection applied to breast MRI: assessment of CAD-generated enhancement and tumor sizes in breast cancers before and after neoadjuvant chemotherapy. Acad Radiol. 2005. 12:806–814.24. Wang LC, DeMartini WB, Partridge SC, Peacock S, Lehman CD. MRI-detected suspicious breast lesions: predictive values of kinetic features measured by computer-aided evaluation. AJR Am J Roentgenol. 2009. 193:826–831.25. Lehman CD, Peacock S, DeMartini WB, Chen X. A new automated software system to evaluate breast MR examinations: improved specificity without decreased sensitivity. AJR Am J Roentgenol. 2006. 187:51–56.26. American College of Radiology. BIRADS: ultrasound. Breast imaging reporting and data system: BIRADS atlas. 2003. 4th ed. Reston, VA: American College of Radiology.27. Suzuki C, Jacobsson H, Hatschek T, Torkzad MR, Bodén K, Eriksson-Alm Y, et al. Radiologic measurements of tumor response to treatment: practical approaches and limitations. Radiographics. 2008. 28:329–344.28. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986. 1:307–310.29. Rieber A, Brambs HJ, Gabelmann A, Heilmann V, Kreienberg R, Kühn T. Breast MRI for monitoring response of primary breast cancer to neo-adjuvant chemotherapy. Eur Radiol. 2002. 12:1711–1719.30. Partridge SC, Gibbs JE, Lu Y, Esserman LJ, Tripathy D, Wolverton DS, et al. MRI measurements of breast tumor volume predict response to neoadjuvant chemotherapy and recurrence-free survival. AJR Am J Roentgenol. 2005. 184:1774–1781.31. Hylton N, Blurne J, Bernreuter W, Pisano E, Rosen M, Morris E. MRI assessment of breast cancer response to neoadjuvant chemotherapy: Preliminary findings of the American College of Radiology Imaging Network (ACRIN) Trial 6657 [abstr]. Radiological Society of North America scientific assembly and annual meeting program. Available via http://rsna2008.rsna.org/event_display.cfm?em_id=6006524. unpublished data.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of the Accuracy of Mammography, Ultrasonography and MRI for Evaluating Residual Tumor after Neoadjuvant Chemotherapy for Breast Cancer
- RECIST Criteria for Tumor Response in the Patients with Breast Cancer Who Had Neoadjuvant Chemotherapy
- Artificial intelligence in breast ultrasound: application in clinical practice
- Predictive and Prognostic Roles of Pathological Indicators for Patients with Breast Cancer on Neoadjuvant Chemotherapy
- Can CD44+/CD24- Tumor Cells Be Used to Determine the Extent of Breast Cancer Invasion Following Neoadjuvant Chemotherapy?