Korean J Radiol.
2010 Aug;11(4):383-394. 10.3348/kjr.2010.11.4.383.
Contrast-Enhanced MR Imaging of Lymph Nodes in Cancer Patients
- Affiliations
-
- 1Department of Radiology and the Clinical Research Institute, Seoul National University Hospital and the Institute of Radiation Medicine, Seoul National University College of Medicine, Seoul 110-744, Korea. moonwk@radcom.snu.ac.kr
- KMID: 984892
- DOI: http://doi.org/10.3348/kjr.2010.11.4.383
Abstract
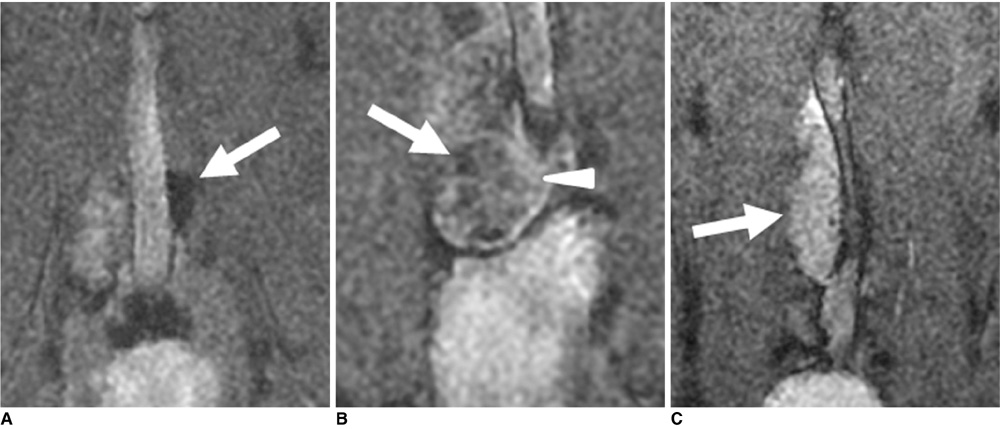
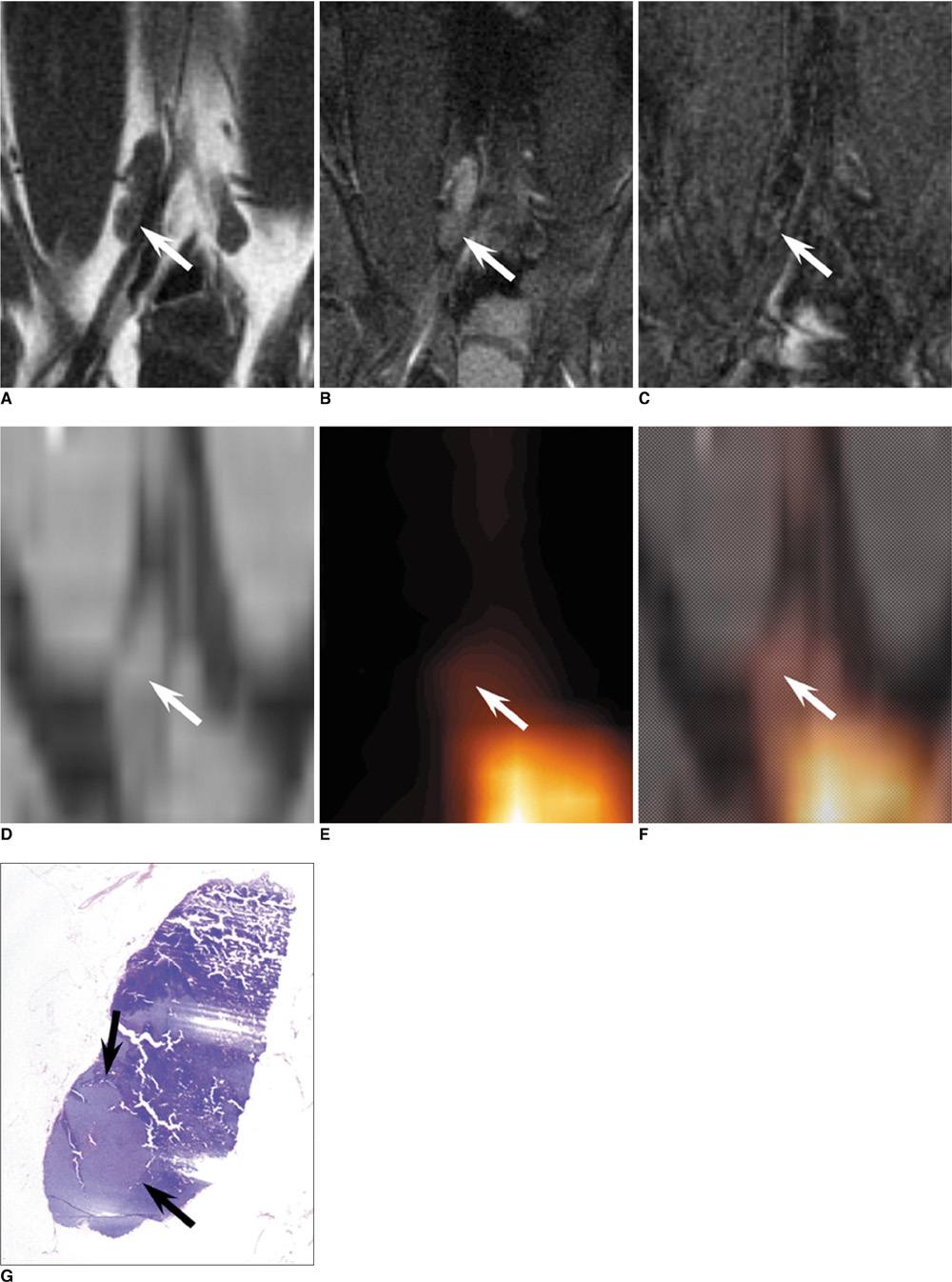
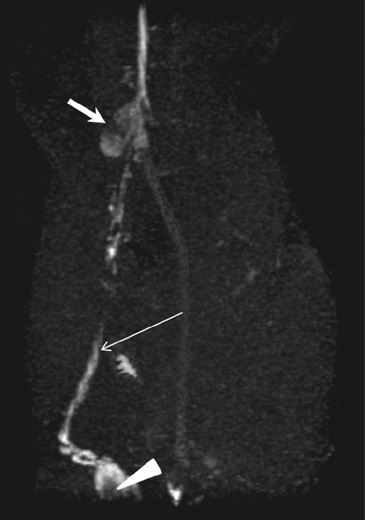
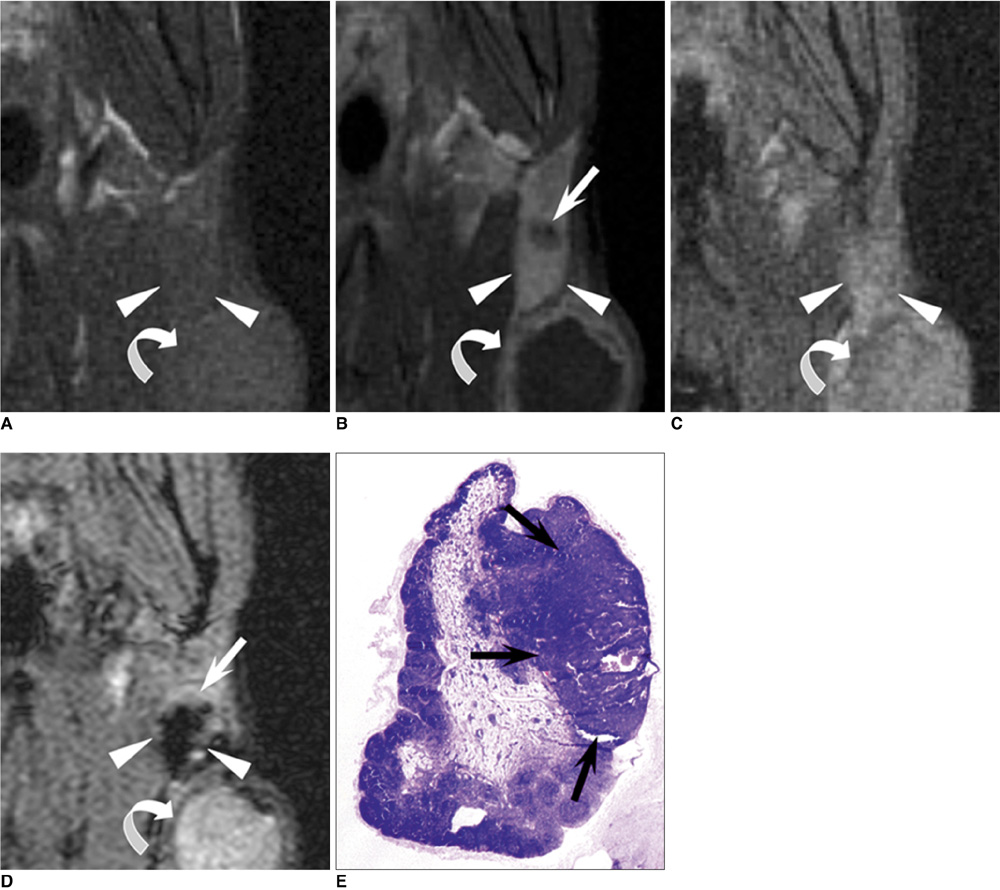
- The accurate identification and characterization of lymph nodes by modern imaging modalities has important therapeutic and prognostic significance for patients with newly diagnosed cancers. The presence of nodal metastases limits the therapeutic options, and it generally indicates a worse prognosis for the patients with nodal metastases. Yet anatomic imaging (CT and MR imaging) is of limited value for depicting small metastatic deposits in normal-sized nodes, and nodal size is a poor criterion when there is no extracapsular extension or focal nodal necrosis to rely on for diagnosing nodal metastases. Thus, there is a need for functional methods that can be reliably used to identify small metastases. Contrast-enhanced MR imaging of lymph nodes is a non-invasive method for the analysis of the lymphatic system after the interstitial or intravenous administration of contrast media. Moreover, some lymphotrophic contrast media have been developed and used for detecting lymph node metastases, and this detection is independent of the nodal size. This article will review the basic principles, the imaging protocols, the interpretation and the accuracies of contrast-enhanced MR imaging of lymph nodes in patients with malignancies, and we also focus on the recent issues cited in the literature. In addition, we discuss the results of several pre-clinical studies and animal studies that were conducted in our institution.
Keyword
MeSH Terms
Figure
Reference
-
1. King AD, Tse GM, Ahuja AT, Yuen EH, Vlantis AC, To EW, et al. Necrosis in metastatic neck nodes: diagnostic accuracy of CT, MR imaging, and US. Radiology. 2004. 230:720–726.2. Anzai Y, Brunberg JA, Lufkin RB. Imaging of nodal metastases in the head and neck. J Magn Reson Imaging. 1997. 7:774–783.3. Gershenwald JE, Thompson W, Mansfield PF, Lee JE, Colome MI, Tseng CH, et al. Multi-institutional melanoma lymphatic mapping experience: the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol. 1999. 17:976–983.4. Laissy JP, Gay-Depassier P, Soyer P, Dombret MC, Murciano G, Sautet A, et al. Enlarged mediastinal lymph nodes in bronchogenic carcinoma: assessment with dynamic contrast-enhanced MR imaging. Work in progress. Radiology. 1994. 191:263–267.5. van den Brekel MW. Lymph node metastases: CT and MRI. Eur J Radiol. 2000. 33:230–238.6. Davis GL. Sensitivity of frozen section examination of pelvic lymph nodes for metastatic prostate carcinoma. Cancer. 1995. 76:661–668.7. Torabi M, Aquino SL, Harisinghani MG. Current concepts in lymph node imaging. J Nucl Med. 2004. 45:1509–1518.8. Moghimi SM, Bonnemain B. Subcutaneous and intravenous delivery of diagnostic agents to the lymphatic system: applications in lymphoscintigraphy and indirect lymphography. Adv Drug Deliv Rev. 1999. 37:295–312.9. Puri SK, Fan CY, Hanna E. Significance of extracapsular lymph node metastases in patients with head and neck squamous cell carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2003. 11:119–123.10. Kapucu LO, Meltzer CC, Townsend DW, Keenan RJ, Luketich JD. Fluorine-18-fluorodeoxyglucose uptake in pneumonia. J Nucl Med. 1998. 39:1267–1269.11. Rennen HJ, Corstens FH, Oyen WJ, Boerman OC. New concepts in infection/inflammation imaging. Q J Nucl Med. 2001. 45:167–173.12. Misselwitz B, Platzek J, Weinmann HJ. Early MR lymphography with gadofluorine M in rabbits. Radiology. 2004. 231:682–688.13. Misselwitz B. MR contrast agents in lymph node imaging. Eur J Radiol. 2006. 58:375–382.14. Witte CL, Williams WH, Witte MH. Lymphatic imaging. Lymphology. 1993. 26:109–111.15. Ruehm SG, Corot C, Debatin JF. Interstitial MR lymphography with a conventional extracellular gadolinium-based agent: assessment in rabbits. Radiology. 2001. 218:664–669.16. Lohrmann C, Földi E, Bartholomä JP, Langer M. MR imaging of the lymphatic system: distribution and contrast enhancement of gadodiamide after intradermal injection. Lymphology. 2006. 39:156–163.17. Misselwitz B, Sachse A. Interstitial MR lymphography using Gd-carrying liposomes. Acta Radiol Suppl. 1997. 412:51–55.18. Weissleder R, Elizondo G, Josephson L, Compton CC, Fretz CJ, Stark DD, et al. Experimental lymph node metastases: enhanced detection with MR lymphography. Radiology. 1989. 171:835–839.19. Harika L, Weissleder R, Poss K, Zimmer C, Papisov MI, Brady TJ. MR lymphography with a lymphotropic T1-type MR contrast agent: Gd-DTPA-PGM. Magn Reson Med. 1995. 33:88–92.20. Staatz G, Nolte-Ernsting CC, Bücker A, Misselwitz B, Grosskortenhaus S, Pflüger D, et al. Interstitial T1-weighted MR lymphography with use of the dendritic contrast agent Gadomer-17 in pigs. Rofo. 2001. 173:1131–1136.21. Misselwitz B, Platzek J, Radüchel B, Oellinger JJ, Weinmann HJ. Gadofluorine 8: initial experience with a new contrast medium for interstitial MR lymphography. MAGMA. 1999. 8:190–195.22. Staatz G, Nolte-Ernsting CC, Adam GB, Grosskortenhaus S, Misselwitz B, Bücker A, et al. Interstitial T1-weighted MR lymphography: lipophilic perfluorinated gadolinium chelates in pigs. Radiology. 2001. 220:129–134.23. Motoyama S, Ishiyama K, Maruyama K, Okuyama M, Sato Y, Hayashi K, et al. Preoperative mapping of lymphatic drainage from the tumor using ferumoxide-enhanced magnetic resonance imaging in clinical submucosal thoracic squamous cell esophageal cancer. Surgery. 2007. 141:736–747.24. Bengele HH, Palmacci S, Rogers J, Jung CW, Crenshaw J, Josephson L. Biodistribution of an ultrasmall superparamagnetic iron oxide colloid, BMS 180549, by different routes of administration. Magn Reson Imaging. 1994. 12:433–442.25. Taupitz M, Wagner S, Hamm B. Contrast media for magnetic resonance tomographic lymph node diagnosis (MR lymphography). Radiologe. 1996. 36:134–140.26. Vassallo P, Matei C, Heston WD, McLachlan SJ, Koutcher JA, Castellino RA. Characterization of reactive versus tumor-bearing lymph nodes with interstitial magnetic resonance lymphography in an animal model. Invest Radiol. 1995. 30:706–711.27. Weissleder R, Heautot JF, Schaffer BK, Nossiff N, Papisov MI, Bogdanov A Jr, et al. MR lymphography: study of a high-efficiency lymphotrophic agent. Radiology. 1994. 191:225–230.28. Weissleder R, Elizondo G, Wittenberg J, Rabito CA, Bengele HH, Josephson L. Ultrasmall superparamagnetic iron oxide: characterization of a new class of contrast agents for MR imaging. Radiology. 1990. 175:489–493.29. Suga K, Yuan Y, Ogasawara N, Okada M, Matsunaga N. Localization of breast sentinel lymph nodes by MR lymphography with a conventional gadolinium contrast agent. Preliminary observations in dogs and humans. Acta Radiol. 2003. 44:35–42.30. Ruehm SG, Schroeder T, Debatin JF. Interstitial MR lymphography with gadoterate meglumine: initial experience in humans. Radiology. 2001. 220:816–821.31. Dimakakos E, Koureas A, Skiadas V, Kostapanagiotou G, Katsenis K, Arkadopoulos N, et al. Interstitial magnetic resonance lymphography with gadobutrol in rabbits and an initial experience in humans. Lymphology. 2006. 39:164–170.32. Trubetskoy VS, Cannillo JA, Milshtein A, Wolf GL, Torchilin VP. Controlled delivery of Gd-containing liposomes to lymph nodes: surface modification may enhance MRI contrast properties. Magn Reson Imaging. 1995. 13:31–37.33. Harika L, Weissleder R, Poss K, Papisov MI. Macromolecular intravenous contrast agent for MR lymphography: characterization and efficacy studies. Radiology. 1996. 198:365–370.34. Kobayashi H, Kawamoto S, Star RA, Waldmann TA, Tagaya Y, Brechbiel MW. Micro-magnetic resonance lymphangiography in mice using a novel dendrimer-based magnetic resonance imaging contrast agent. Cancer Res. 2003. 63:271–276.35. Kobayashi H, Kawamoto S, Sakai Y, Choyke PL, Star RA, Brechbiel MW, et al. Lymphatic drainage imaging of breast cancer in mice by micro-magnetic resonance lymphangiography using a nano-size paramagnetic contrast agent. J Natl Cancer Inst. 2004. 96:703–708.36. Misselwitz B, Schmitt-Willich H, Michaelis M, Oellinger JJ. Interstitial magnetic resonance lymphography using a polymeric t1 contrast agent: initial experience with Gadomer-17. Invest Radiol. 2002. 37:146–151.37. Desser TS, Rubin DL, Muller H, McIntire GL, Bacon ER, Hollister KR. Interstitial MR and CT lymphography with Gd-dTPA-co-alpha, omega-diaminoPEG(1450) and Gd-dTPA-co-1,6-diaminohexane polymers: preliminary experience. Acad Radiol. 1999. 6:112–118.38. Herborn CU, Vogt FM, Lauenstein TC, Goyen M, Dirsch O, Corot C, et al. Assessment of normal, inflammatory, and tumor-bearing lymph nodes with contrast-enhanced interstitial magnetic resonance lymphography: preliminary results in rabbits. J Magn Reson Imaging. 2003. 18:328–335.39. Herborn CU, Lauenstein TC, Vogt FM, Lauffer RB, Debatin JF, Ruehm SG. Interstitial MR lymphography with MS-325: characterization of normal and tumor-invaded lymph nodes in a rabbit model. AJR Am J Roentgenol. 2002. 179:1567–1572.40. Choi SH, Han MH, Moon WK, Son KR, Won JK, Kim JH, et al. Cervical lymph node metastases: MR imaging of gadofluorine M and monocrystalline iron oxide nanoparticle-47 in a rabbit model of head and neck cancer. Radiology. 2006. 241:753–762.41. Cha JH, Moon WK, Cheon JE, Koh YH, Lee EH, Park SS, et al. Differentiation of hyperplastic from metastatic lymph nodes using a lymph node specific MR contrast agent gadofluroine M. J Korean Radiol Soc. 2006. 55:183–189. [Korean].42. Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003. 348:2491–2499.43. Choi SH, Moon WK, Hong JH, Son KR, Cho N, Kwon BJ, et al. Lymph node metastasis: ultrasmall superparamagnetic iron oxide-enhanced MR imaging versus PET/CT in a rabbit model. Radiology. 2007. 242:137–143.44. Weissleder R, Elizondo G, Wittenberg J, Lee AS, Josephson L, Brady TJ. Ultrasmall superparamagnetic iron oxide: an intravenous contrast agent for assessing lymph nodes with MR imaging. Radiology. 1990. 175:494–498.45. Mühler A, Zhang X, Wang H, Lawaczeck R, Weinmann HJ. Investigation of mechanisms influencing the accumulation of ultrasmall superparamagnetic iron oxide particles in lymph nodes. Invest Radiol. 1995. 30:98–103.46. Bush CH, Mladinich CR, Montgomery WJ. Evaluation of an ultrasmall superparamagnetic iron oxide in MRI in a bone tumor model in rabbits. J Magn Reson Imaging. 1997. 7:579–584.47. Rogers JM, Jung CW, Lewis J, Groman EV. Use of USPIO-induced magnetic susceptibility artifacts to identify sentinel lymph nodes and lymphatic drainage patterns. I. Dependence of artifact size with subcutaneous Combidex dose in rats. Magn Reson Imaging. 1998. 16:917–923.48. Bellin MF, Lebleu L, Meric JB. Evaluation of retroperitoneal and pelvic lymph node metastases with MRI and MR lymphangiography. Abdom Imaging. 2003. 28:155–163.49. Harisinghani MG, Dixon WT, Saksena MA, Brachtel E, Blezek DJ, Dhawale PJ, et al. MR lymphangiography: imaging strategies to optimize the imaging of lymph nodes with ferumoxtran-10. Radiographics. 2004. 24:867–878.50. Safety Data from Study 38804-10: A phase III safety and efficacy study of combidex as a magnetic resonance imaging agent for the evaluation of lymph node disease, AMAG. Boston, MA, USA: Pharmaceuticals, Inc..51. Islam T, Harisinghani MG. Overview of nanoparticle use in cancer imaging. Cancer Biomark. 2009. 5:61–67.52. Will O, Purkayastha S, Chan C, Athanasiou T, Darzi AW, Gedroyc W, et al. Diagnostic precision of nanoparticle-enhanced MRI for lymph-node metastases: a meta-analysis. Lancet Oncol. 2006. 7:52–60.53. Anzai Y, Piccoli CW, Outwater EK, Stanford W, Bluemke DA, Nurenberg P, et al. Evaluation of neck and body metastases to nodes with ferumoxtran 10-enhanced MR imaging: phase III safety and efficacy study. Radiology. 2003. 228:777–788.54. Saksena M, Harisinghani M, Hahn P, Kim J, Saokar A, King B, et al. Comparison of lymphotropic nanoparticle-enhanced MRI sequences in patients with various primary cancers. AJR Am J Roentgenol. 2006. 187:W582–W588.55. Narayanan P, Iyngkaran T, Sohaib SA, Reznek RH, Rockall AG. Pearls and pitfalls of MR lymphography in gynecologic malignancy. Radiographics. 2009. 29:1057–1069.56. Kimura K, Tanigawa N, Matsuki M, Nohara T, Iwamoto M, Sumiyoshi K, et al. High-resolution MR lymphography using ultrasmall superparamagnetic iron oxide (USPIO) in the evaluation of axillary lymph nodes in patients with early stage breast cancer: preliminary results. Breast Cancer. 2009. [Epub ahead of print].57. Heesakkers RA, Fütterer JJ, Hövels AM, van den Bosch HC, Scheenen TW, Hoogeveen YL, et al. Prostate cancer evaluated with ferumoxtran-10-enhanced T2*-weighted MR Imaging at 1.5 and 3.0 T: early experience. Radiology. 2006. 239:481–487.58. Choi SH, Kim KH, Moon WK, Kim HC, Cha JH, Paik JH, et al. Comparison of lymph node metastases assessment with the use of USPIO-enhanced MR imaging at 1.5 T versus 3.0 T in a rabbit model. J Magn Reson Imaging. 2010. 31:134–141.59. Lahaye MJ, Engelen SM, Kessels AG, de Bruïne AP, von Meyenfeldt MF, van Engelshoven JM, et al. USPIO-enhanced MR imaging for nodal staging in patients with primary rectal cancer: predictive criteria. Radiology. 2008. 246:804–811.60. Lee AS, Weissleder R, Brady TJ, Wittenberg J. Lymph nodes: microstructural anatomy at MR imaging. Radiology. 1991. 178:519–522.61. Koh DM, Brown G, Temple L, Raja A, Toomey P, Bett N, et al. Rectal cancer: mesorectal lymph nodes at MR imaging with USPIO versus histopathologic findings--initial observations. Radiology. 2004. 231:91–99.62. Anzai Y, Prince MR. Iron oxide-enhanced MR lymphography: the evaluation of cervical lymph node metastases in head and neck cancer. J Magn Reson Imaging. 1997. 7:75–81.63. Anzai Y, Blackwell KE, Hirschowitz SL, Rogers JW, Sato Y, Yuh WT, et al. Initial clinical experience with dextran-coated superparamagnetic iron oxide for detection of lymph node metastases in patients with head and neck cancer. Radiology. 1994. 192:709–715.64. Bellin MF, Roy C, Kinkel K, Thoumas D, Zaim S, Vanel D, et al. Lymph node metastases: safety and effectiveness of MR imaging with ultrasmall superparamagnetic iron oxide particles--initial clinical experience. Radiology. 1998. 207:799–808.65. Sigal R, Vogl T, Casselman J, Moulin G, Veillon F, Hermans R, et al. Lymph node metastases from head and neck squamous cell carcinoma: MR imaging with ultrasmall superparamagnetic iron oxide particles (Sinerem MR) -- results of a phase-III multicenter clinical trial. Eur Radiol. 2002. 12:1104–1113.66. Stets C, Brandt S, Wallis F, Buchmann J, Gilbert FJ, Heywang-Köbrunner SH. Axillary lymph node metastases: a statistical analysis of various parameters in MRI with USPIO. J Magn Reson Imaging. 2002. 16:60–68.67. Mack MG, Balzer JO, Straub R, Eichler K, Vogl TJ. Superparamagnetic iron oxide-enhanced MR imaging of head and neck lymph nodes. Radiology. 2002. 222:239–244.68. Deserno WM, Harisinghani MG, Taupitz M, Jager GJ, Witjes JA, Mulders PF, et al. Urinary bladder cancer: preoperative nodal staging with ferumoxtran-10-enhanced MR imaging. Radiology. 2004. 233:449–456.69. Rockall AG, Sohaib SA, Harisinghani MG, Babar SA, Singh N, Jeyarajah AR, et al. Diagnostic performance of nanoparticle-enhanced magnetic resonance imaging in the diagnosis of lymph node metastases in patients with endometrial and cervical cancer. J Clin Oncol. 2005. 23:2813–2821.70. Stadnik TW, Everaert H, Makkat S, Sacré R, Lamote J, Bourgain C. Breast imaging. Preoperative breast cancer staging: comparison of USPIO-enhanced MR imaging and 18F-fluorodeoxyglucose (FDC) positron emission tomography (PET) imaging for axillary lymph node staging--initial findings. Eur Radiol. 2006. 16:2153–2216.71. Memarsadeghi M, Riedl CC, Kaneider A, Galid A, Rudas M, Matzek W, et al. Axillary lymph node metastases in patients with breast carcinomas: assessment with nonenhanced versus uspio-enhanced MR imaging. Radiology. 2006. 241:367–377.72. Heesakkers RA, Hövels AM, Jager GJ, van den Bosch HC, Witjes JA, Raat HP, et al. MRI with a lymph-node-specific contrast agent as an alternative to CT scan and lymph-node dissection in patients with prostate cancer: a prospective multicohort study. Lancet Oncol. 2008. 9:850–856.73. Fischbein NJ, Noworolski SM, Henry RG, Kaplan MJ, Dillon WP, Nelson SJ. Assessment of metastatic cervical adenopathy using dynamic contrast-enhanced MR imaging. AJNR Am J Neuroradiol. 2003. 24:301–311.74. Lee KC, Moon WK, Chung JW, Choi SH, Cho N, Cha JH, et al. Assessment of lymph node metastases by contrast-enhanced MR imaging in a head and neck cancer model. Korean J Radiol. 2007. 8:9–14.75. Murray AD, Staff RT, Redpath TW, Gilbert FJ, Ah-See AK, Brookes JA, et al. Dynamic contrast enhanced MRI of the axilla in women with breast cancer: comparison with pathology of excised nodes. Br J Radiol. 2002. 75:220–228.76. Luciani A, Dao TH, Lapeyre M, Schwarzinger M, Debaecque C, Lantieri L, et al. Simultaneous bilateral breast and high-resolution axillary MRI of patients with breast cancer: preliminary results. AJR Am J Roentgenol. 2004. 182:1059–1067.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pelvic Lymph Node Evaluation in Uterine Cervical Carcinoma Using Contrast Enhanced MR Imaging
- Cervical lymph node pathology on lymphatic enhanced CT scan
- Supraclavicular Lymph Node Metastasis from Various Malignancies: Assessment with 18F-Fluorodeoxyglucose Positron Emission Tomography/CT, Contrast-Enhanced CT and Ultrasound
- Assessment of Lymph Node Metastases by Contrast-Enhanced MR Imaging in a Head and Neck Cancer Model
- (18)F-FDG PET/CT with Contrast Enhancement for Evaluation of Axillary Lymph Node Involvement in T1 Breast Cancer