Korean J Ophthalmol.
2010 Aug;24(4):240-244. 10.3341/kjo.2010.24.4.240.
Two Cases of Corneal Ulcer due to Methicillin-Resistant Staphylococcus aureus in High Risk Groups
- Affiliations
-
- 1Department of Ophthalmology and Visual Science, The Catholic University of Korea School of Medicine, Seoul, Korea. mskim@catholic.ac.kr
- 2HanGil Eye Hospital, Incheon, Korea.
- KMID: 949063
- DOI: http://doi.org/10.3341/kjo.2010.24.4.240
Abstract
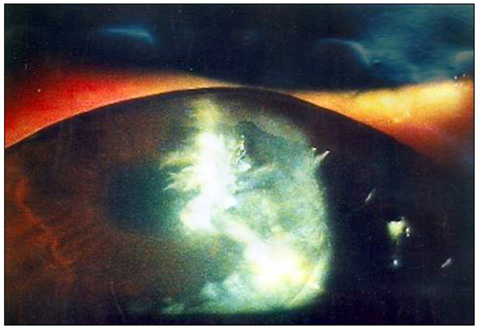
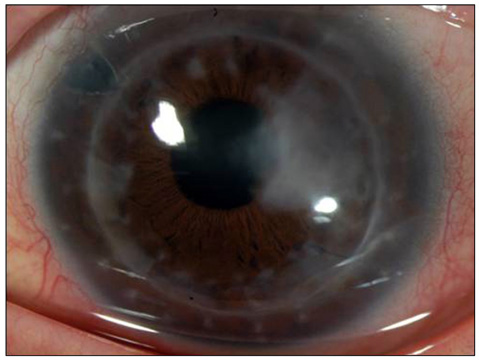
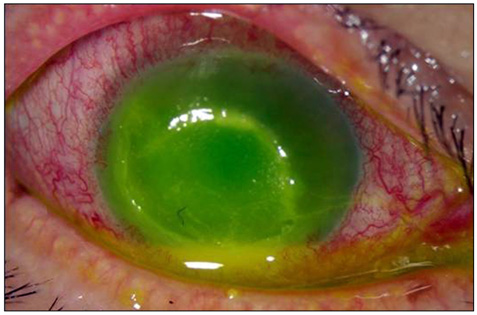
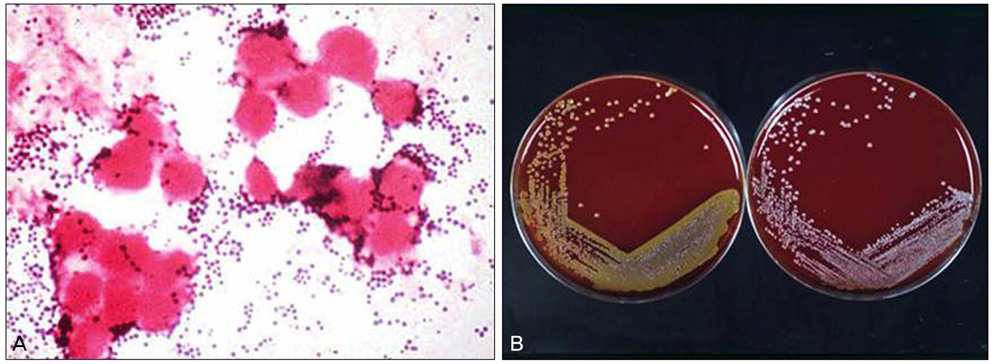
- Considering the popular use of antibiotic-containing eyedrops in Korea, it is important to know the emerging antibiotic-resistant strains of bacteria before treating infectious eye diseases. This is especially important in high-risk groups because of the high incidence of resistant infections and the subsequent treatment requirements. We report two cases of methicillin-resistant Staphylococcus aureus (MRSA) corneal ulcers in high-risk groups. The first case involved a patient who had keratitis after using antibiotic- and steroid-containing eyedrops to treat a corneal opacity that developed after repeated penetrating keratoplasty. The second case involved a patient who used antibiotic-containing eyedrops and a topical lubricant on a regular basis for >1 month to treat exposure keratitis due to lagophthalmos. The second patient's problems, which included a persistent superficial infiltration, developed after brain tumor surgery. Both cases showed MRSA on corneal culture, and the corneal ulcers improved in both patients after the application of vancomycin-containing eyedrops. In conclusion, MRSA infection should be considered in corneal ulcers that have a round shape, mild superficial infiltration, and slow progression, especially in high-risk groups. This report includes descriptions of the characteristic features, antibiotic sensitivities, prevention, and successful treatment with vancomycin-containing eyedrops for MRSA corneal ulcers.
MeSH Terms
-
Cornea/*microbiology/pathology
Corneal Ulcer/diagnosis/*microbiology
Diagnosis, Differential
Eye Infections, Bacterial/diagnosis/*microbiology
Female
Follow-Up Studies
Humans
Male
Methicillin-Resistant Staphylococcus aureus/*isolation & purification
Middle Aged
Staphylococcal Infections/diagnosis/*microbiology
Figure
Reference
-
1. Brumfitt W, Hamilton-Miller J. Methicillin-resistant Staphylococcus aureus. N Engl J Med. 1989. 320:1188–1196.2. Kirby WM. Extraction of a highly potent penicillin inactivator from penicillin resistant staphylococci. Science. 1944. 99:452–453.3. Rolinson GN, Stevens S, Batchelor FR, et al. Bacteriological studies on a new penicillin-BRL. 1241. Lancet. 1960. 2:564–567.4. Jevons MP. "Celbenin"-resistant Staphylococci. Br Med J. 1961. 1:124–125.5. Knox R. "Celbenin"-resistant Staphylococci. Br Med J. 1961. 1:126.6. Sahm DF, Marsilio MK, Piazza G. Antimicrobial resistance in key bloodstream bacterial isolates: electronic surveillance with the Surveillance Network Database: USA. Clin Infect Dis. 1999. 29:259–263.7. Diekema DJ, Pfaller MA, Schmitz FJ, et al. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin Infect Dis. 2001. 32:Suppl 2. S114–S132.8. Sotozono C, Inagaki K, Fujita A, et al. Methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylococcus epidermidis infections in the cornea. Cornea. 2002. 21:S94–S101.9. Kim IS, Shin YM, Kim SJ, et al. Comparison of antimicrobial efficacy of topical antibiotics in rabbit keratitis model with ciprofloxacin, methicillin-resistant Staphylococcus aureus. J Korean Ophthalmol Soc. 2000. 41:807–814.10. Insler MS, Fish LA, Silbernagel J, et al. Successful treatment of methicillin-resistant Staphylococcus aureus Keratitis with topical ciprofloxacin. Ophthalmology. 1991. 98:1690–1692.11. Snyder ME, Katz HR. Ciprofloxacin-resistant bacterial keratitis. Am J Ophthalmol. 1992. 114:336–338.12. Harbarth S, Francois P, Shrenzel J, et al. Community-associated methicillin-resistant Staphylococcus aureus, Switzerland. Emerg Infect Dis. 2005. 11:962–965.13. Charlebois ED, Bangsberg DR, Moss NJ, et al. Population-based community prevalence of methicillin-resistant Staphylococcus aureus in the urban poor of San Francisco. Clin Infect Dis. 2002. 34:425–433.14. Pan ES, Diep BA, Charlebois ED, et al. Population dynamics of nasal strains of methicillin-resistant Staphylococcus aureus and their relation to community-associated disease activity. J Infect Dis. 2005. 192:811–818.15. Blomquist PH. Methicillin-resistant Staphylococcus aureus infections of the eye and orbit (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2006. 104:322–345.16. Maple PA, Hamilton-Miller JM, Brumfitt W. World-wide antibiotic resistance in methicillin-resistant Staphylococcus aureus. Lancet. 1989. 1:537–540.17. Brennen C, Muder RR. Conjunctivitis associated with methicillin-resistant Staphylococcus aureus in a long-term-care facility. Am J Med. 1990. 88:14N–17N.18. Mulligan ME, Murray-Leisure KA, Ribner BS, et al. Methicillin-resistant Staphylococcus aureus: a consensus review of the microbiology, pathogenesis, and epidemiology with implications for prevention and management. Am J Med. 1993. 94:313–328.19. Herwaldt LA. Control of methicillin-resistant Staphylococcus aureus in the hospital setting. Am J Med. 1999. 106:11S–18S.20. Pittet D, Hugonnet S, Harbarth S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene: Infection Control Programme. Lancet. 2000. 356:1307–1312.21. Fleischer AB, Hoover DL, Khan JA, et al. Topical vancomycin formulation for methicillin-resistant Staphylococcus epidermidis blepharoconjunctivitis. Am J Ophthalmol. 1986. 101:283–287.22. Forster W, Becker K, Hungermann D, Busse H. Methicillin-resistant Staphylococcus aureus keratitis after excimer laser photorefractive keratectomy 1. J Cataract Refract Surg. 2002. 28:722–724.23. Goodman DF, Gottsch JD. Methicillin-resistant Staphylococcus epidermidis keratitis treated with vancomycin. Arch Ophthalmol. 1988. 106:1570–1571.24. Khan JA, Hoover D, Ide CH. Methicillin-resistant Staphylococcus epidermidis blepharitis. Am J Ophthalmol. 1984. 98:562–565.25. Shanmuganathan VA, Armstrong M, Buller A, Tullo AB. External ocular infections due to methicillin-resistant Staphylococcus aureus (MRSA). Eye (Lond). 2005. 19:284–291.26. Rutar T, Chambers HF, Crawford JB, et al. Ophthalmic manifestations of infections caused by the USA300 clone of community-associated methicillin-resistant Staphylococcus aureus. Ophthalmology. 2006. 113:1455–1462.27. Kato T, Hayasaka S. Methicillin-resistant Staphylococcus aureus and methicillin-resistant coagulase-negative staphylococci from conjunctivas of preoperative patients. Jpn J Ophthalmol. 1998. 42:461–465.28. Mitsuda T, Arai K, Fujita S, Yokota S. Epidemiological analysis of strains of methicillin-resistant Staphylococcus aureus (MRSA) infection in the nursery: prognosis of MRSA carrier infants. J Hosp Infect. 1995. 31:123–134.29. Cooper BS, Stone SP, Kibbler CC, et al. Isolation measures in the hospital management of methicillin resistant Staphylococcus aureus (MRSA): systematic review of the literature. BMJ. 2004. 329:533.30. Boyce JM, Potter-Bynoe G, Chenevert C, King T. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol. 1997. 18:622–627.31. Devine J, Cooke RP, Wright EP. Is methicillin-resistant Staphylococcus aureus (MRSA) contamination of ward-based computer terminals a surrogate marker for nosocomial MRSA transmission and handwashing compliance? J Hosp Infect. 2001. 48:72–75.32. Layton MC, Perez M, Heald P, Patterson JE. An outbreak of mupirocin-resistant Staphylococcus aureus on a dermatology ward associated with an environmental reservoir. Infect Control Hosp Epidemiol. 1993. 14:369–375.33. Dietze B, Rath A, Wendt C, Martiny H. Survival of MRSA on sterile goods packaging. J Hosp Infect. 2001. 49:255–261.34. Muto CA, Jernigan JA, Ostrowsky BE, et al. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and enterococcus. Infect Control Hosp Epidemiol. 2003. 24:362–386.35. Suh Y, Shin EM, Hahn TW. Corneal ulcer and chronic conjunctivitis due to ofloxacin-resistant MRSA. J Korean Ophthalmol Soc. 2002. 43:419–423.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Detection of Multidrug Resistant Patterns and Associated - genes of Methicillin Resistant Staphylococcus aureus ( MRSA ) Isolated from Clinical Specimens
- A statistical analysis of methicillin-resistant staphylococcus aureus
- A case of multiple furunculosis caused by methicillin-resistant staphylococcs aureus
- Corneal Ulcer and Chronic Conjunctivitis Due to Ofloxacin-resistant MRSA
- Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA)