Korean J Ophthalmol.
2009 Jun;23(2):93-99. 10.3341/kjo.2009.23.2.93.
Innervated Myotendinous Cylinders Alterations in Human Extraocular Muscles in Patients With Strabismus
- Affiliations
-
- 1Department of Ophthalmology, Eulji University School of Medicine, Eulji General Hospital, Seoul, Korea.
- 2Department of Ophthalmology, Sungkyunkwan University School of Medicine, Samsung Medical Center, Seoul, Korea. syoh@skku.edu
- KMID: 754724
- DOI: http://doi.org/10.3341/kjo.2009.23.2.93
Abstract
-
PURPOSE: To analyze innervated myotendinous cylinders (IMCs) in the extraocular muscles (EOMs) of normal subjects and strabismic patients.
METHODS
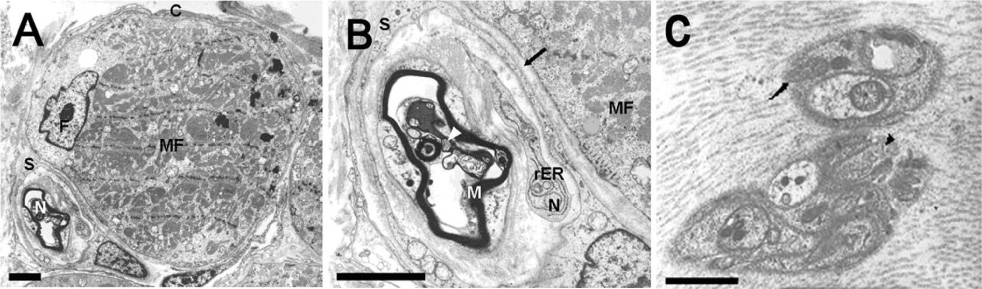
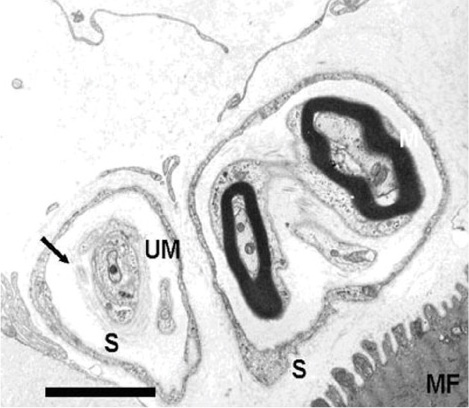
The rectus muscles of 37 subjects were analyzed. Distal myotendinous specimens were obtained from 3 normal subjects, 20 patients with acquired strabismus, 11 with infantile strabismus, and from 3 with congenital nystagmus, and were studied by using light microscopy. Some specimens (6 rectus muscles) were also examined by transmission electron microscopy.
RESULTS
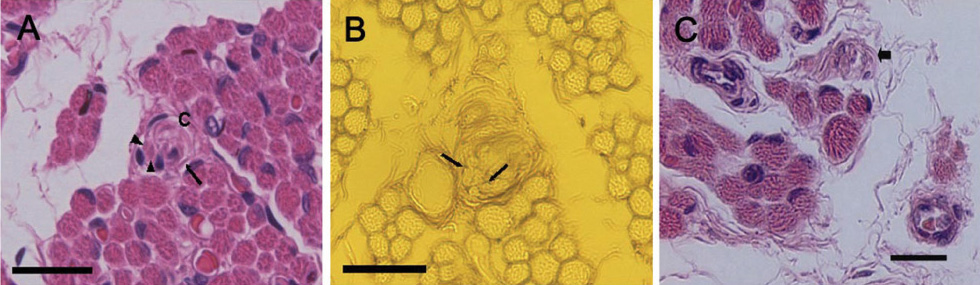
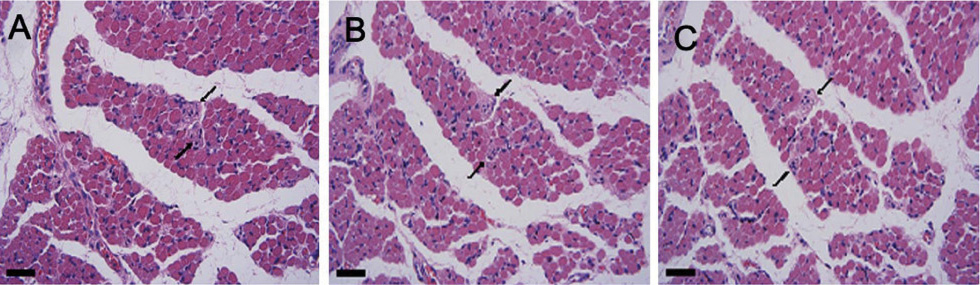
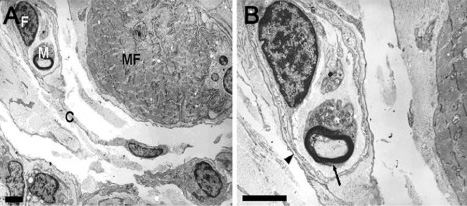
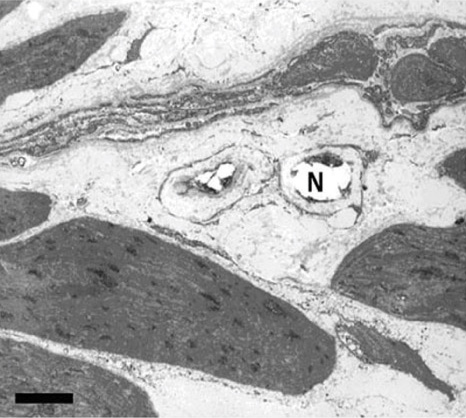
IMCs were found in the distal myotendinous regions of EOMs. The IMCs of patients with acquired strabismus showed no significant morphological alterations. However, significant IMCs alterations were observed at the distal myotendinous junction of patients with congenital strabismus and congenital nystagmus.
CONCLUSIONS
This study supports the notion that IMCs in human EOMs function mainly as proprioceptors, along with effector properties, and a disturbance of ocular proprioceptors plays an important role in the pathogenesis of oculomotor disorder. We suggest that a proprioceptive feedback system should be stimulated and calibrated early in life for the development of binocular vision.
Keyword
MeSH Terms
Figure
Reference
-
1. Fiorentini A, Berardi N, Maffei L. Role of extraocular proprioception in the orienting behavior of cats. Exp Brain Res. 1982. 48:113–120.2. Gauthier GM, Nommay D, Vercher J-L. The role of ocular muscle proprioception in visual localization of targets. Science. 1990. 249:58–61.3. Bridgeman B, Stark L. Ocular proprioception and efference copy in registering visual direction. Vision Res. 1991. 31:1903–1913.4. Buisseret P. Influence of extraocular muscle proprioception on vision. Physiol Rev. 1995. 75:323–338.5. Steinbach MJ, Smith DR. Spatial localization after strabismus surgery: evidence for inflow. Science. 1981. 213:1407–1409.6. Tamura O, Mitsui Y. The magician's forceps phenomenon in exotropia under general anesthesia. Br J Ophthalmol. 1986. 70:549–552.7. Corsi M, Sodi A, Salvi G, Faussone-Pellegrini MS. Morphological study of extraocular muscle proprioceptor alterations in congenital strabismus. Ophthalmologica. 1990. 200:154–163.8. Bruenech JR, Rusckell GL. Muscle spindles in extraocular muscles of human infants. Cell Tissues Organs. 2001. 169:388–394.9. Ruskell GL. The fine structure of human extraocular muscle spindles and their potential proprioceptive capacity. J Anat. 1989. 167:199–214.10. Bruenech JR, Ruskell GL. Extraocular muscle spindles in infants. In: Papers presented at the meeting of The Society for Experimental Optometry, in Birmingham; UK, on 27-28 July 1992. Ophthal Physiol Opt. 1993. 13:103–110.11. Lukas JR, Aigner M, Blumer R, et al. Number and distribution of neuromuscular spindles in human extraocular muscle. Invest Ophthalmol Vis Sci. 1994. 35:4317–4327.12. Blummer R, Lukas JR, Aigner M, et al. Fine structural analysis of extraocular muscle spindles of a two-year-old human infant. Invest Ophthalmol Vis Sci. 1999. 40:55–64.13. Richmond FJ, Johnston WS, Baker RS, Steinbach MJ. Palisade endings in human extraocular muscles. Invest Ophthalmol Vis Sci. 1984. 25:471–476.14. Sodi A, Corsi M, Faussone-Pellegrini MS, Salvi G. Fine structure of the receptors at the myotendinous junction of human extraocular muscles. Histol Histopathol. 1988. 3:103–113.15. Dogiel AS. Die Endigungen der sensiblen Nerven in den Augenmuskeln und deren Sehnen beim Menschen und den Säugetieren. Arch Mikr Anat Entwick. 1906. 68:501–526.16. Ruskell GL. The fine structure of innervated myotendinous cylinders in extraocular muscles of rhesus monkey. J Neurocytol. 1978. 7:693–708.17. Bérard-Badier M, Pellisser JF, Toga M, et al. Ultrastructural studies of extraocular muscles in ocular motility disorders. Albrecht Von Grafes Arch Klin Exp Ophthalmol. 1978. 208:193–205.18. Spencer RF, McNeer KW. Structural alterations in overacting inferior oblique muscles. Arch Ophthalmol. 1980. 98:128–133.19. Martinez A, Biglan AW, Hiles DA. Structural features of extraocular muscles of children with strabismus. Arch Ophthalmol. 1980. 98:533–539.20. Domenici-Lombardo L, Corsi M, Mencucci R, et al. Extraocular muscles in congenital strabismus: muscle fiber and nerve ending ultrastructure according to different regions. Ophthalmologica. 1992. 205:29–39.21. Bruenech R, Ruskell GL. Myotendinous nerve endings in human infant and adult extraocular muscles. Anat Rec. 2000. 260:132–140.22. Ruskell GL. Extraocular muscle proprioceptors and proprioception. Prog Retin Eye Res. 1999. 18:269–291.23. Lukas JR, Blumer R, Denk M, et al. Innervated myotendinous cylinders in human extraocular muscles. Invest Ophthalmol Vis Sci. 2000. 41:2422–2431.24. Alvarado-Mallart RM, Pinçon-Raymond M. The palisade endings of cat extraocular muscles: a light and electron microscope study. Tissue Cell. 1979. 11:567–584.25. Porter JD, Spencer RF. Localization and morphology of cat extraocular muscle afferent neurons identified by retrograde transport of horseradish peroxidase. J Comp Neurol. 1982. 204:56–64.26. Billig I, Buisseret-Delmas C, Buisseret P. Identification of nerve endings in cat extraocular muscle. Anat Rec. 1997. 248:566–575.27. Donaldson IML. The functions of proprioceptors of the eye muscles. Philos Trans R Soc Lond B Biol Sci. 2000. 355:1685–1754.28. Buettner-Ennever JA, Horn AK. The anatomical basis of oculomotor disorders: the dual motor control of extraocualr muscles and its possible role in proprioception. Curr Opin Neurol. 2002. 15:35–43.29. Buettner-Ennever JA, Eberhorn A, Horn AK. Motor and sensory innervation of extraocular eye muscles. Ann NY Acad Sci. 2003. 1004:40–49.30. Sas J, Scháb R. Die sogenannten. "Palisaden-Endigungen" der Augenmuskeln. Acta Morph Acad Sci Hung. 1952. 2:259–266.31. Blumer R, Wasicky R, Hoetzenecker W, Lukas JR. Presence and structure of innervated myotendinous cylinders in rabbit extraocular muscle. Exp Eye Res. 2001. 73:787–796.32. Konakci KZ, Stricher J, Hoetznecker W, et al. Molecular characteristics suggest an effector function of palisade endings in extraocualr muscles. Invest Ophthalmol Vis Sci. 2005. 46:155–165.33. Hertle RW, Chan CC, Galita DA, et al. Neuroanatomy of the extraocular muscle tendon enthesis in macaque, normal human, and patients with congenital nystagmus. J AAPOS. 2002. 6:319–327.34. Soukup T, Zelena J. Structure of tendon organs of the rat after neonatal de-efferentation. Cell Tissue Res. 1985. 241:229–236.35. Zelená J, Hnik P. Motor and receptor units in the sole muscle after nerve regeneration in very young rats. Physiol bohemoslov. 1963. 12:277–290.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ultrastructural Changes of Myotendinous Nerve Endings following Recession or Resection Procedures of Extraocular Muscle Surgeries in Cats
- The Morphological Differences of Proprioceptors in Extraocular Muscles among Congenital, Acquired Exotropia and Congenital Nystagmus
- Oculocardiac Reflex During Strabismus Surgery
- Oculocardiac Reflex during Strabismus Surgery
- Surgical Correction of Lid Retraction with a Silicone Sponge in Congenital Fibrosis of the Extraocular Muscles