Korean J Ophthalmol.
2004 Dec;18(2):121-131. 10.3341/kjo.2004.18.2.121.
Effect of Basic Fibroblast Growth Factor-Saporin (bFGF-SAP) Conjugate on Bovine Choriocapillary Endothelial Cells
- Affiliations
-
- 1Department of Ophthalmology, St. Paul's Hospital, Catholic University Medical College, Korea.
- 2Department of Ophthalmology, Kangnam St. Mary's Hospital, Catholic University Medical College, Korea.
- 3Open St. Mary Eye Center, Seoul, Korea.
- KMID: 754377
- DOI: http://doi.org/10.3341/kjo.2004.18.2.121
Abstract
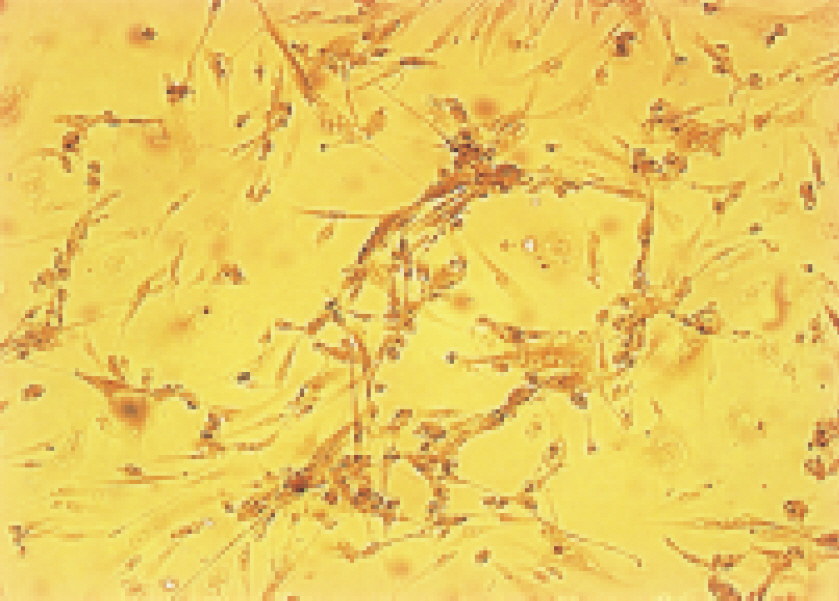
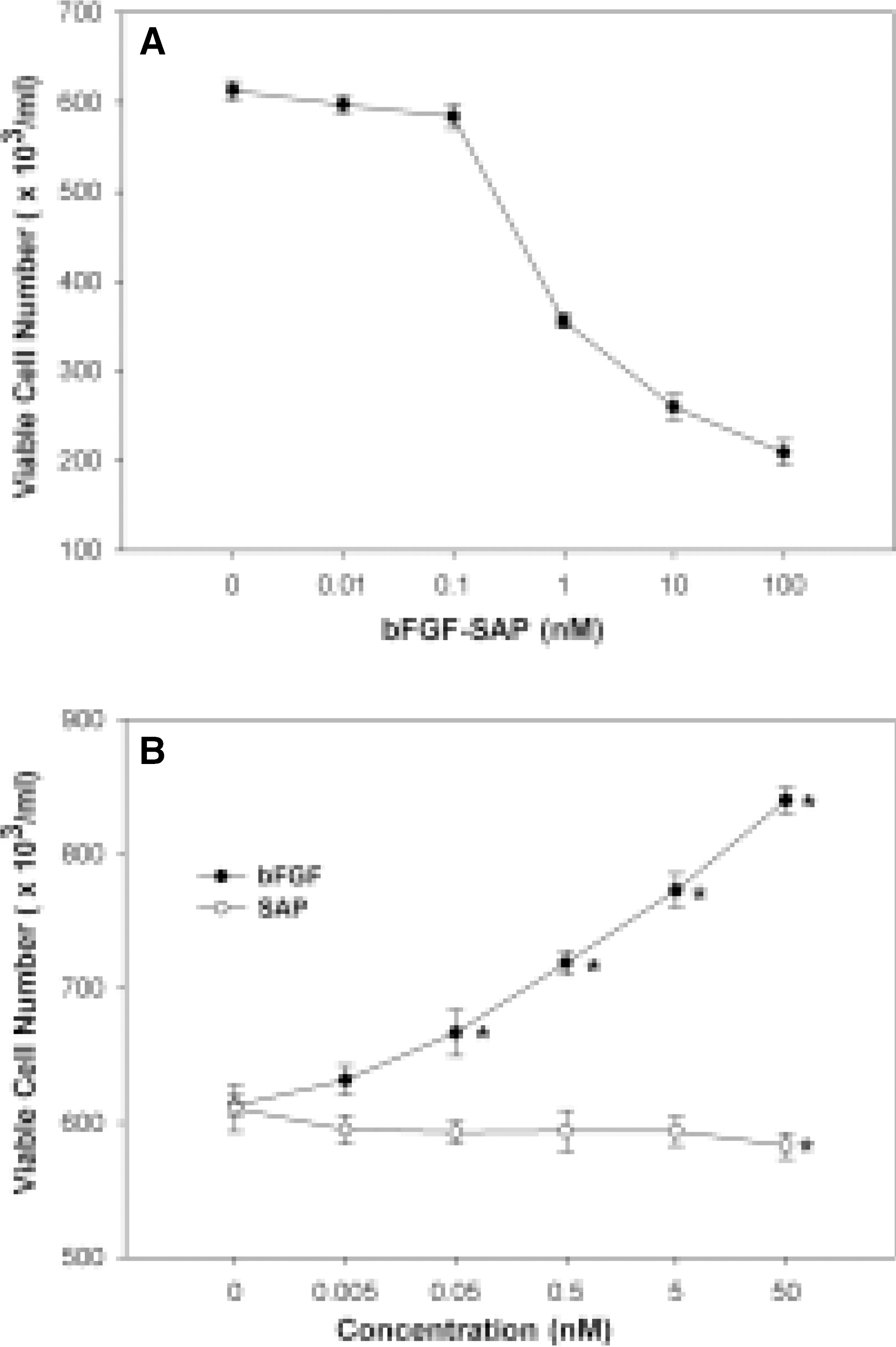
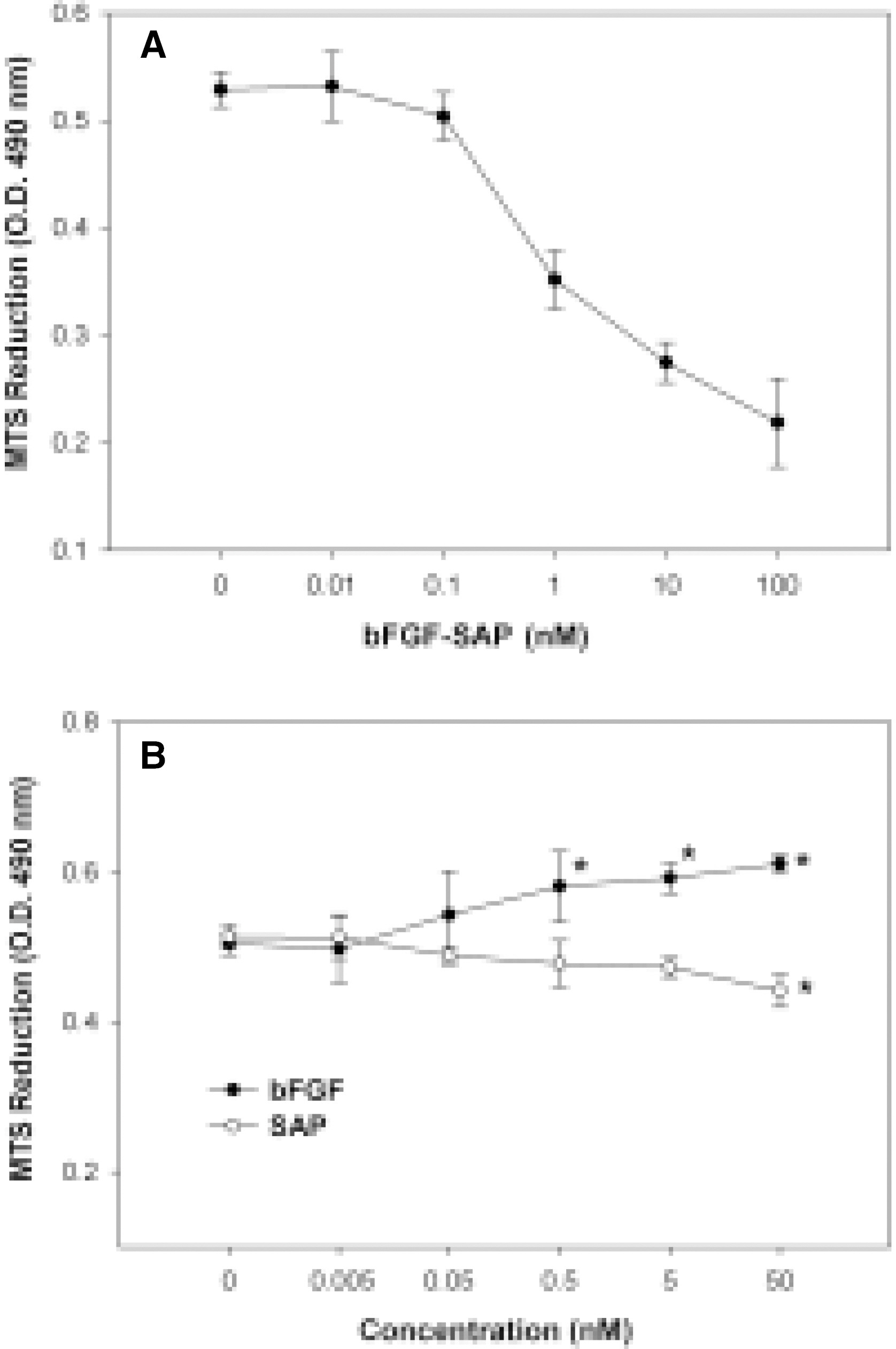
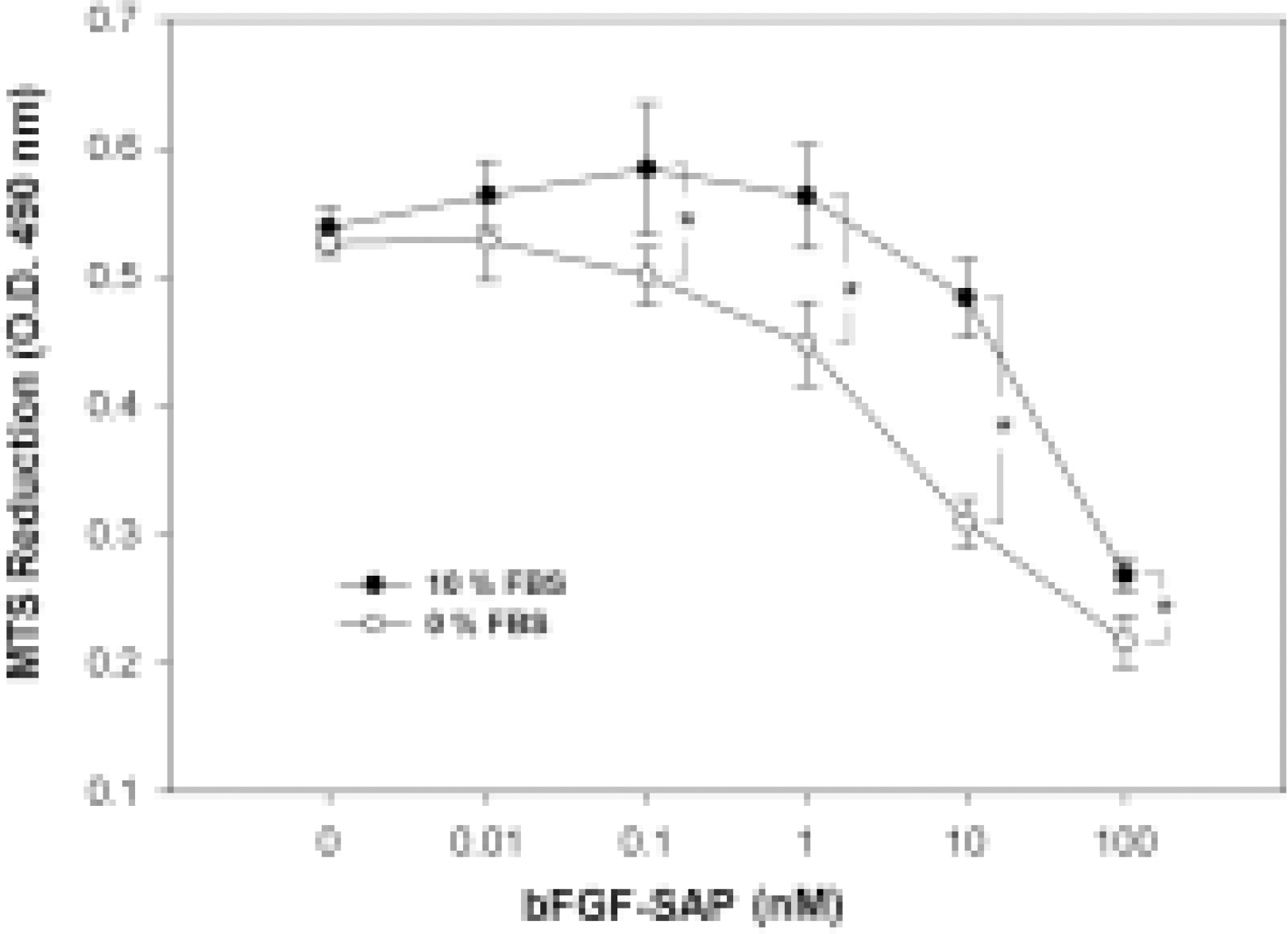
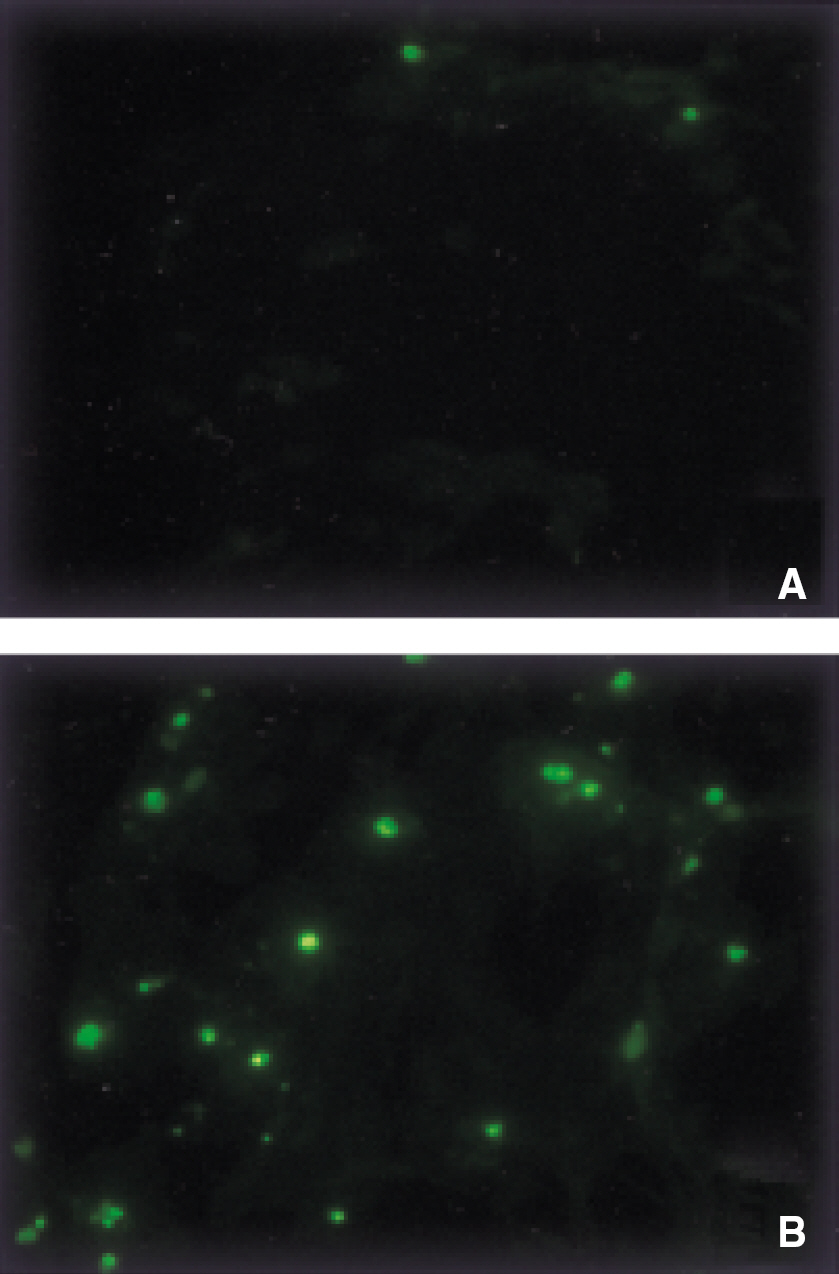
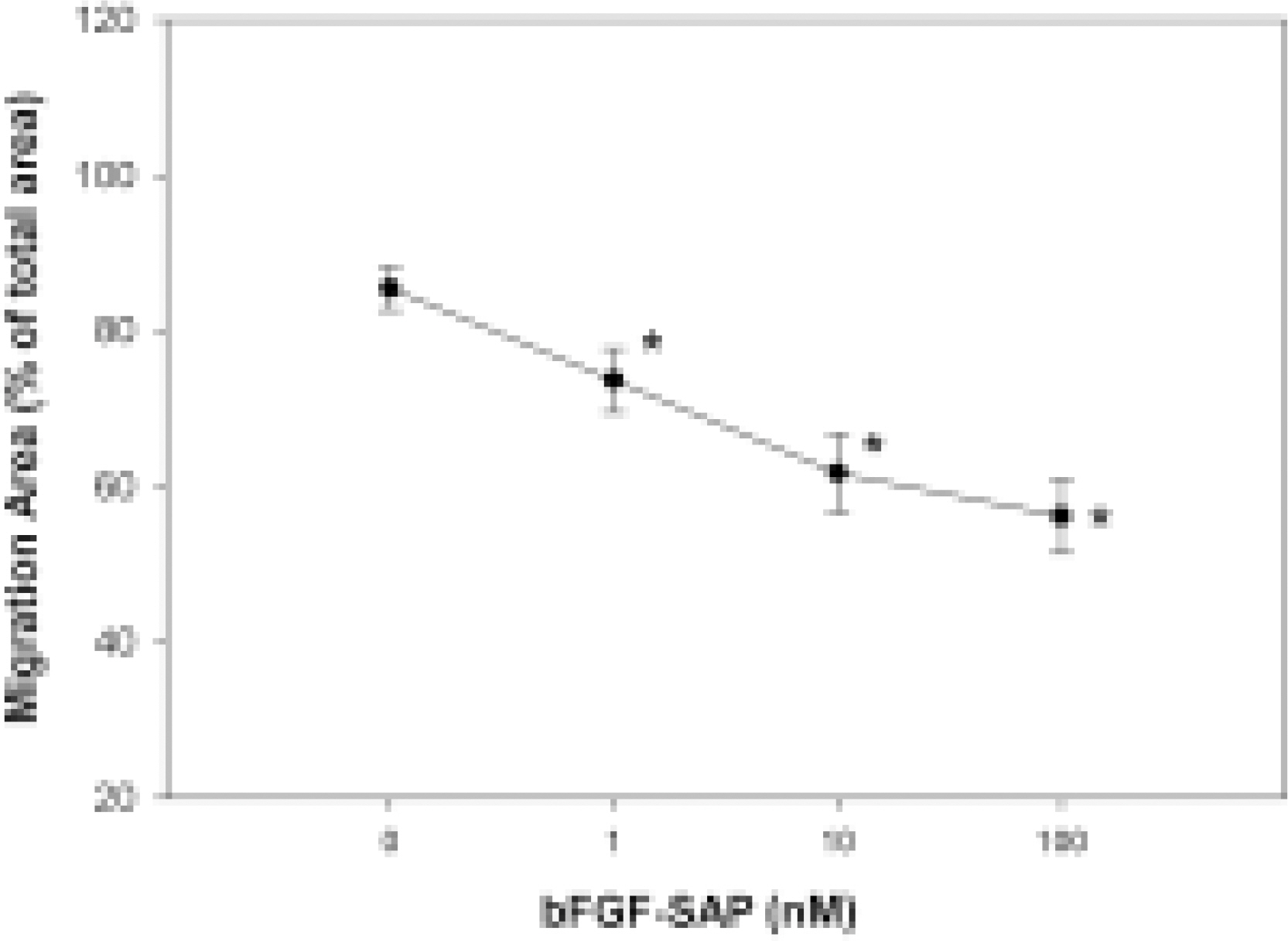
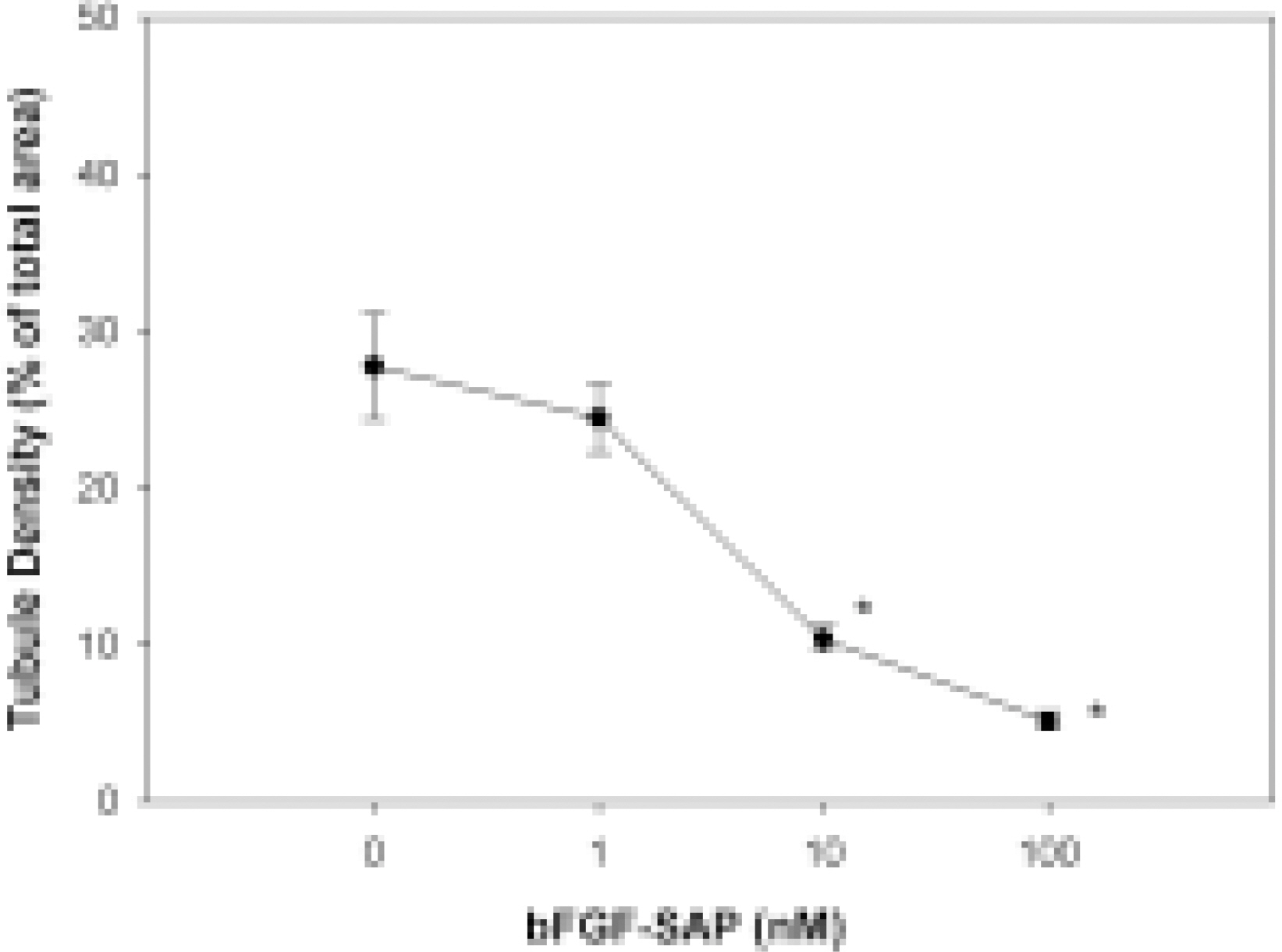
- We evaluated the effect of a basic fibroblast growth factor (bFGF) and saporin conjugate (bFGF-SAP) on proliferation, migration and tubule formation in bovine choriocapillary endothelial cells (BCECs). Cell proliferation and MTS assays were done with 0.01, 0.1, 1, 10, and 100 nM bFGF-SAP, and an equimolar concentration of bFGF and saporin. TUNEL assay was performed to confirm apoptosis. Cells were treated with 1, 10, and 100 nM bFGF-SAP and migration assay and tubule formation assay were done. Results were evaluated with image analysis. All experiments were performed in triplicate and repeated three times. Viable cells (ID50 = 0.62) and cell proliferation by MTS assay (ID50 = 0.75 nM) were inhibited. Saporin caused cytotoxicity and inhibition of proliferation at high concentration. DNA fragmentation was identified by TUNEL assay. Migration and tubule formation were also inhibited. All mechanisms responsible for neovascularization were inhibited, and this could be applied in the management of subretinal choroidal neovascularization (SRN).
Keyword
MeSH Terms
-
Animals
Apoptosis/drug effects
Cattle
Cell Count
Cell Movement/drug effects
Cell Proliferation/drug effects
Cells, Cultured
Comparative Study
Cytotoxins/*pharmacology
Endothelium, Vascular/*drug effects
Fibroblast Growth Factor 2/*pharmacology
In Situ Nick-End Labeling
Neovascularization, Physiologic/drug effects
Plant Proteins/*pharmacology
Recombinant Fusion Proteins/*pharmacology
Figure
Reference
-
References
1. Sarks SH. New vessel formation beneath the retinal pigment epithelium in senile eye. Br J Ophthalmol. 1973; 57:951–965.2. Teeters VW, Bird AC. A clinical study of the vascularity of senile disciform macular degeneration. Am J Ophthalmol. 1973; 75:53–65.
Article3. Ryan SJ, Mittl RN, Maumenee AE. The disciform response: an historical perspective. Graefe's Arch Clin Exp Ophthalmol. 1980; 215:1–20.
Article4. Green WR, Wilson DJ. Choroidal neovascularization. Ophthalmology. 1986; 93:1169–1176.
Article5. Macular Photocoagulation Study Group. Argon laser photocoagulation for neovascular maculopathy after five years. Arch Ophthalmol. 1991; 109:1109–1114.6. Frank RN, Amin RH, Puklin JE. Antioxidant enzymes in the macular retinal pigment epithelium of eyes with neovascular age-related macular degeneration. Am J Ophthalmol. 1999; 127:694–709.7. Jampol LM. Antioxidants, zinc, and age-related macular degeneration: results and recommendations. Arch Ophthalmol. 2001; 119:1533–1534.8. Jampol LM, Ferris FL 3rd. Antioxidants and zinc to prevent progression of age-related macular degeneration. JAMA. 2001; 286:2466–2468.9. Schmidt-Erfurth U, Miller J, Sickenberg M, Bunse A, Laqua H, Gragoudas E, Zografos L, Birngruber R, van den Bergh H, Strong A, Manjuris U, Fsadni M, Lane AM, Piguet B, Bressler NM. Photodynamic therapy of subfoveal choroidal neovascularization: clinical and angiographic examples. Graefe's Arch Clin Exp Ophthalmol. 1998; 236:365–374.
Article10. Ladd BS, Solomon SD, Bressler NM, Bressler SB. Photodynamic therapy with verteporfin for choroidal neovascularization in patients with diabetic retinopathy. Am J Ophthalmol. 2001; 132:659–667.
Article11. Bressler NM. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization-verteporfin in photodynamic therapy report 2. Am J Ophthalmol. 2002; 133:168–169.
Article12. Pastan I, Willingham MC, Fitzgerald DJP. Immunotoxins. Cell. 1986; 47:641–648.
Article13. Beitz JG, Davol P, Clark JW, Kato J, Medina M, Frackelton AR, Lappi DA, Baird A, Calabresi P. Antitumor activity of basic fibroblast growth factor-saporin mitotoxin in vitro and vivo. Cancer Res. 1992; 52:227–230.14. Siena S, Lappi DA, Bregni M, Formosa A, Villa S, Soria M, Bonadonna G, Gianni AM. Synthesis and characterization of an antihuman T-lymphocyte saporin immunotoxin (OKT1-SAP) with in vivo stability into nonhuman primates. Blood. 1988; 72:756–765.
Article15. Santanche S, Belleli A, Brunori M. The unusual stability of saporin, a candidate for the synthesis of immunotoxins. Biochem Biophys Res Commun. 1997; 234:129–132.16. Lappi DA, Martineau D, Baird A. Biological and chemical characterization of basic FGF-saporin mitotoxin. Biochem Biophys Res Commun. 1989; 160:917–923.
Article17. Lappi DA, Martineau DG, Nakamura S, Buscaglia M, Baird A. Basic fibroblast growth factor-saporin mitotoxin: an endothelial cell growth inhibitor. J Cell Biochem. 1990; 14E(Suppl.):222.18. Lappi DA, Maher PA, Martineau D, Baird A. The basic fibroblast growth factor mitotoxin acts through the basic fibroblast growth factor. J Cell Physiol. 1991; 147:17–26.19. Axel DI, Riessen R, Runge H, Viebahn R, Karsch KR. Effects of cerivastatin on human arterial smooth muscle cell proliferation and migration in transfilter cocultures. J Cardiovasc Pharmacol. 2000; 35:619–629.
Article20. Lehr HA, van der Loos CM, Teeling P, Gown AM. Complete chromogen separation and analysis in double immunohistochemical stains using Photoshop-based image analysis. J Histochem Cytochem. 1999; 47:119–126.
Article21. Matsumura T, Wolff K, Petzelbauer P. Endothelial cell tube formation depends on cadherin 5 and CD31 interactions with filamentous actin. J Immunol. 1997; 158:3408–3416.22. Murata T, He S, Hangai M, Ishibashi T, Xi XP, Kim S, Hsueh WA, Ryan SJ, Law RE, Hinton DR. Peroxisome proliferator-activated receptor-gamma ligands inhibit choroidal neovascularization. Invest Ophthalmol Vis Sci. 2000; 41:2309–2317.23. Yamakawa K, Bhutto IA, Lu Z, Watanabe Y, Amemiya T. Retinal vascular changes in rats with inherited hypercholesterolemia – corrosion cast demonstration. Curr Eye Res. 2001; 22:258–265.
Article24. Kobayashi H, Kobayashi K. Radiotherapy for subfoveal neovascularisation associated with pathological myopia: a pilot study. Br J Ophthalmol. 2000; 84:761–766.
Article25. Cho J, Jun BK, Lee YJ, Uhm KB. Factors associated with the poor final visual outcome after traumatic hyphema. Korean J Ophthalmol. 1998; 12:122–129.
Article26. Lim JI. Iatrogenic choroidal neovascularization. Surv Ophthalmol. 1999; 44:95–111.
Article27. Hargrave S, Weakley D, Wilson C. Complications of ocular paintball injuries in children. J Pediatr Ophthalmol Strabismus. 2000; 37:338–343.
Article28. Eckstein M, Wells JA, Aylward B, Gregor Z. Surgical removal of non-age-related subfoveal choroidal neovascular membranes. Eye. 1998; 12:775–780.
Article29. Wolf S, Lappas A, Weinberger AW, Kirchhof B. Macular translocation for surgical management of subfoveal choroidal neovascularizations in patients with AMD: first results. Graefe's Arch Clin Exp Ophthalmol. 1999; 237:51–57.
Article30. Jaakkola A, Heikkonen J, Tarkkanen A, Immonen I. Visual function after strontium-90 plaque irradiation in patients with age-related subfoveal choroidal neovascularization. Acta Ophthalmol Scand. 1999; 77:57–61.
Article31. Flaxel CJ, Friedrichsen EJ, Smith JO, Oeinck SC, Blacharski PA, Garcia CA, Chu HH. Proton beam irradiation of subfoveal choroidal neovascularisation in age-related macular degeneration. Eye. 2000; 14:155–164.
Article32. Davol P, Beitz JG, Mohler M, Ying W, Cook J, Lappi DA, Frackelton AR. Saporin toxins directed to basic fibroblast growth factor receptors effectively target human ovarian teratocarcinoma in an animal model. Cancer. 1995; 76:79–85.
Article33. Davol P, Frackelton AR. The mitotoxin, basic fibroblast growth factor-saporin, effectively targets human prostatic carcinoma in an animal model. J Urol. 1996; 156:1174–1179.
Article34. Chandler LA, Sosnowski BA, McDonald JR, Price JE, Aukerman SL, Baird A, Pierce GF, Houston LL. Targeting tumor cells via EGF receptors: selective toxicity of an HBEGF-toxin fusion protein. Int J Cancer. 1998; 78:106–111.35. Sforzini S, de Totero D, Gaggero A, Ippoliti R, Glennie MJ, Canevari S, Stein H, Ferrini S. Targeting of saporin to Hodgkin's lymphoma cells by anti-CD30 and anti-CD25 bispecific antibodies. Br J Haematol. 1998; 102:1061–1068.
Article36. Pastan I, Kreitman RJ. Immunotoxins for targeted cancer therapy. Adv Drug Deliv Rev. 1998; 31:53–88.37. Davol PA, Frackelton AR. Targeting human prostatic carcinoma through basic fibroblast growth factor receptors in an animal model: characterizing and circumventing mechanisms of tumor resistance. Prostate. 1999; 40:178–191.
Article38. Scott CF, Lambert JM, Goldmacher VS, Blattler WA, Sobel R, Schlossman SF, Benacerraf B. The pharmacokinetics and toxicity of murine monoclonal antibodies and of gelonin conjugates of these antibodies. Int J Immunopharmacol. 1987; 9:211–225.
Article39. Ghetie V, Vitetta E. Immunotoxins in the therapy of cancer: from bench to clinic. Pharmac Ther. 1994; 63:209–234.
Article40. Bolognesi A, Tazzari PL, Olivieri F, Polito L, Falini B, Stirpe F. Induction of apoptosis by ribosome-inactivating proteins and related immunotoxins. Int J Cancer. 1996; 68:349–355.
Article41. Behar-Cohen FF, David T, D'Hermies F, Pouliquen YM, Buechler Y, Nova MP, Houston LL, Courtois Y. In vivo inhibition of lens regrowth by fibroblast growth factor 2-saporin. Invest Ophthalmol Vis Sci. 1995; 36:2434–2448.42. Wee WR, Parandoosh Z, Sakamoto T, Caton M, Nova M, McDonnell PJ. Antiproliferative effect of basic fibroblast growth factor-saporin mitotoxin on keratocytes in culture. Korean J Ophthalmol. 1996; 10:1–7.
Article43. Bretton RH, Swearingen A, Kash RL, Cooley R. Use of a polylysine-saporin conjugate to prevent posterior capsule opacification. J Cataract Refract Surg. 1999; 25:921–929.
Article44. Jaffe EA. Cell biology of endothelial cells. Hum Pathol. 1987; 18:234–239.
Article45. Kumar S, West DC, Ager A. Heterogeneity in endothelial cells from large vessels and microvessels. Differentiation. 1987; 36:57–70.
Article46. Fajardo LF. The complexity of endothelial cells: a review. Am J Clin Pathol. 1989; 92:241–250.47. Page C, Rose M, Yacoub M, Pigott R. Antigenic heterogeneity of vascular endothelium. Am J Pathol. 1992; 141:673–683.
Article48. Hoffmann S, Spee C, Murata T, Cui JZ, Ryan SJ, Hinton DR. Rapid isolation of choriocapillary endothelial cells by Lycopersicon esculentum-coated Dynabeads. Graefe's Arch Clin Exp Ophthalmol. 1998; 236:779–784.49. Baird A. Fibroblast Growth Factors: What's in a name? Endocrinology. 1993; 132:487–488.
Article50. Goto F, Goto K, Weindel K, Folkman J. Synergistic effects of vascular endothelial growth factor and basic fibroblast growth factor on the proliferation and cord formation of bovine capillary endothelial cells within collagen gels. Lab Invest. 1993; 69:508–517.51. Stirpe F, Bailey S, Miller SP, Bodley JW. Modification of ribosomal RNA by ribosome-inactivating proteins from plants. Nucleic Acids Res. 1988; 16:1349–1357.
Article52. Perkins AC, Pimm MV, Baldwin RW. Demonstration of the hepatic uptake of radiolabelled immunotoxins using gamma scintigraphy. Eur J Cancer Clin Oncol. 1987; 23:1225–1227.
Article53. Bergamaschi G, Cazzola M, Dezza L, Savino E, Consonni L, Lappi D. Killing of K562 cells with conjugates between human transferrin and a ribosome-inactivating protein (SO6). Br J Haematol. 1988; 68:379–384.
Article54. Lappi DA, Baird A. Mitotoxins: Growth factor-targeted cytotoxic molecules. Progress in Growth Factor Research. 1990; 2:223–236.
Article55. Bergamaschi G, Perfetti V, Tonon L, Novella A, Lucotti C, Danova M, Glennie MJ, Merlini G, Cazzola M. Saporin, a ribosome-inactivating protein used to prepare immunotoxins, induces cell death via apoptosis. Br J Haematol. 1996; 93:789–794.
Article56. Morse LS, Sidikaro Y. Isolation and characterization of bovine choroidal microvessel endothelium and pericytes in culture. Curr Eye Res. 1990; 9:631–642.
Article57. Chapman N, Witt N, Gao X, Bharath AA, Stanton AV, Thom SA, Hughes AD. Computer algorithms for the automated measurement of retinal arteriolar diameters. Br J Ophthalmol. 2001; 85:74–79.
Article58. Sakamoto T, Sakamoto H, Murphy TL, Spee Christine, Soriano D, Ishibashi T, Hinton DR, Ryan SJ. Vessel formation by choroidal endothelial cells in vitro is modulated by retinal pigment epithelial cells. Arch Ophthalmol. 1995; 113:512–520.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antiproliferative effect of basic fibroblast growth factor-saporin mitotoxin on keratocytes in culture
- The Effect of Vascular Endothelial Growth Factor and basic Fibroblast Growth Factor on the Growth of Endometrial Stromal Sarcoma
- Vascular Endothelial Growth Factor and basic Fibroblast Growth Factor Expression in Early Gastric Carcinomas: Correlation with Clinicopathologic Factors
- Clinical Significance of Basic Fibroblast Growth Factor Expression in Bladder Cancer
- The Effects of TGF-beta2 and bFGF on the Proliferation of Retinal Pigment Epithelial Cells