Korean J Radiol.
2003 Mar;4(1):27-34. 10.3348/kjr.2003.4.1.27.
Percutaneous Radiofrequency Thermal Ablation with Hypertonic Saline Injection: In Vivo Study in a Rabbit Liver Model
- Affiliations
-
- 1Department of Radiology, Seoul National University College of Medicine, Seoul, Korea. leejm@radcom.snu.ac.kr
- 2Department of Radiology, Chonbuk National University Medical School, Chonju, Korea.
- 3Department of Radiology, Wonkwang University Hospital, Iksan, Korea.
- KMID: 754039
- DOI: http://doi.org/10.3348/kjr.2003.4.1.27
Abstract
OBJECTIVE
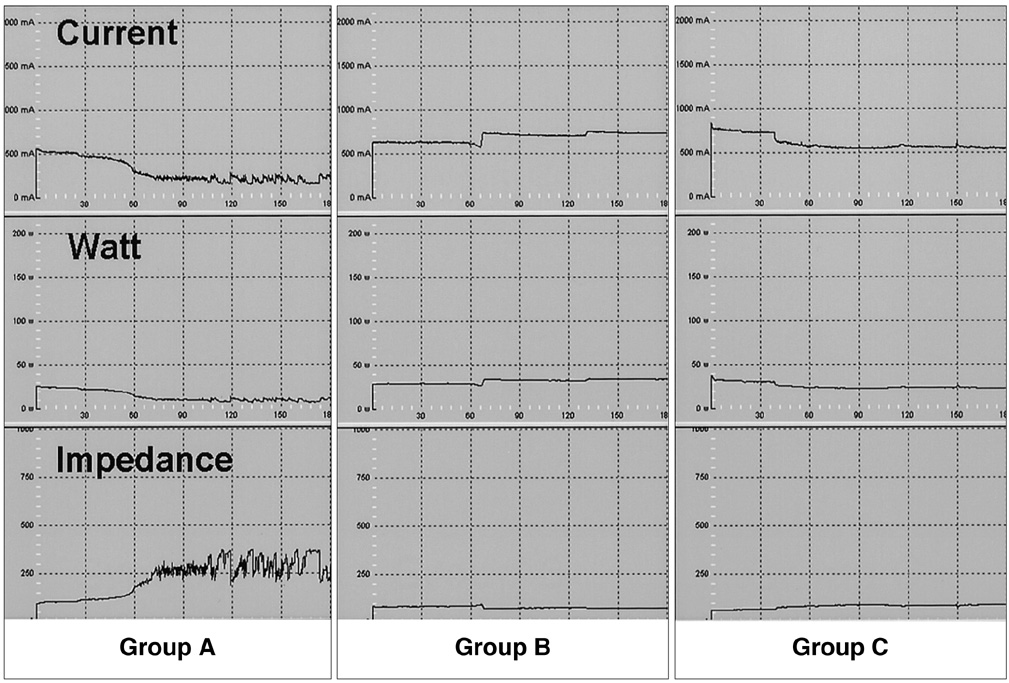
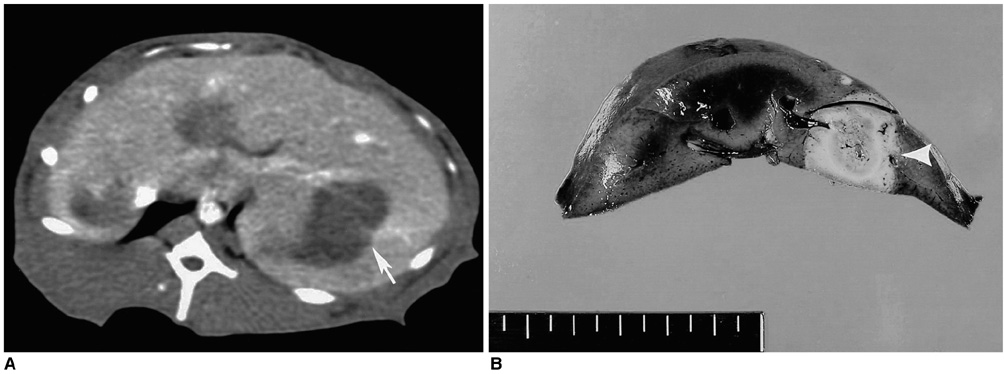
To determine whether hypertonic saline (HS, 36% NaCl) injection prior to or during radiofrequency ablation (RFA) can increase the extent of thermally mediated coagulation in in-vivo rabbit liver tissue, and also to establish the ideal injection time in relation to RFA in order to maximize its effect on the extent of radiofrequency (RF) -induced coagulation. MATERIALS AND METHODS: In 26 rabbits, 43 RFA lesions were produced using a 17-gauge internally cooled electrode with a 1-cm active tip under ultrasound (US) guidance. Rabbits were assigned to one of three groups: Group A: RFA alone (n=8) ; Group B: RFA after the instillation of 1 mL HS (n=8) ; Group C: RFA after and during the instillation of 0.5 mL HS (n=10). RF energy (30 W) was applied for 3 minutes, and changes occurring in tissue impedance, current, power output, and the temperature of the electrode tip were automatically measured. After RFA, contrast-enhanced spiral CT was performed, and in each group the maximum diameters of the thermal lesions in gross specimens were compared. Technical success and the complications arising were evaluated by CT and on the basis of autopsy findings. RESULTS: All procedures were technically successful. There were six procedure-related complications (6/26; 23%), including five localized perihepatic hematomas and one thermal injury to the stomach. With instillation of HS in group B rabbits, markedly decreased tissue impedance (73 omega+/-5) and increased current (704 mA+/-41) were noted, compared to RF ablation without saline infusion (116.3 omega+/-13, 308 mA+/-80). With instillation of the solution before RFA (group B), coagulation necrosis was greater (14.9 mm+/- 3.8) than in rabbits not injected (group A: 11.5 mm+/-2.4; Group A vs. B: p < .05) and in those injected before and during RFA (group C: 12.5 mm+/-3.1; Group B vs. C: p > .05). CONCLUSION: RFA using HS instillation can increase the volume of RFAinduced necrosis of the liver with a single application, thereby simplifying and accelerating the treatment of larger lesions. In addition, HS instillation before RFA more effectively achieves coagulation necrosis than HS instillation before and during RFA.
Figure
Reference
-
1. Lim HK. Radiofrequency thermal ablation of hepatocellular carcinomas. Korean J Radiol. 2000. 1:175–184.2. Curley SA, Izzo F, Ellis LM, Vauthey JN, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000. 232:381–391.3. Curley SA, Izzo F, Delrio P, Ellis LM, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999. 230:1–8.4. Rossi S, Di Stasi M, Buscarini E, et al. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol. 1996. 167:759–768.5. Goldberg SN, Livraghi T, Solbiati L, Gazelle GS. Gazelle GS, Saini S, Mueller PR, editors. In-situ ablation of focal hepatic neoplasms. Hepatobiliary and pancreatic radiology: imaging and intervention. 1997. New York: Thieme;470–502.6. Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. Tumor ablation with radiofrequency energy. Radiology. 2000. 217:633–646.7. Gervais DA, McGovern FJ, Wood BJ, Goldberg SN, McDougal WC, Mueller PR. Radio-frequency ablation of renal cell carcinoma: early clinical experience. Radiology. 2000. 217:665–672.8. Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998. 80:815–821.9. Jeffrey SS, Birdwell RL, Ikeda DM, et al. Radiofrequency ablation of breast cancer: first report of an emerging technology. Arch Surg. 1999. 134:1064–1068.10. Anzai Y, Lufkin R, DeSalles A, Hamilton DR, Farahani K, Black KL. Preliminary experience with MR-guided thermal ablation of brain tumors. AJNR Am J Neuroradiol. 1995. 16:39–48.11. Livraghi T, Goldberg SN, Lazzaroni S, et al. Hepatocellular carcinoma: radiofrequency ablation of medium and large lesions. Radiology. 2000. 214:761–768.12. de Baere T, Elias D, Dromain C, et al. Radiofrequency ablation of 100 hepatic metastases with a mean follow-up of more than one year. AJR Am J Roentgenol. 2000. 175:1619–1625.13. Dodd GD, Frank MS, Aribandi M, Chopra S, Chintapalli KN. Radiofrequency thermal ablation: computer analysis created by overlapping ablations. AJR Am J Roentgenol. 2002. 177:777–782.14. Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol. 2000. 174:323–331.15. Solbiati L, Goldberg SN, Ierace T, et al. Hepatic metastases: percutaneous radiofrequency ablation with cooled-tip electrodes. Radiology. 1997. 205:367–373.16. McGahan JP, Gu WZ, Brock JM, Tesluk H, Jones CD. Hepatic ablation using bipolar radiofrequency electrocautery. Acad Radiol. 1996. 3:418–422.17. Munver R, Threatt CB, Delvecchio FC, Preminger GM, Polascik TJ. Hypertonic saline-augmented radiofrequency ablation of VX2 tumor implanted in rabbit kidney: a short-term survival pilot study. Urology. 2002. 60:170–175.18. Livraghi T, Goldberg SN, Monti F, et al. Saline-enhanced radiofrequency tissue ablation in the treatment of liver metastases. Radiology. 1997. 202:205–210.19. Goldberg SN, Ahmed M, Gazelle GS, et al. Radiofrequency thermal ablation with NaCl solution injection: effect of electrical conductivity on tissue heating and coagulation-phantom and porcine liver study. Radiology. 2001. 219:157–165.20. Ahmed M, Lobo SM, Weinstein J, et al. Improved coagulation with saline solution pretreatment during radiofrequency tumor ablation in a canine model. J Vasc Interv Radiol. 2002. 13:717–724.21. Miao Y, Ni Y, Yu J, Marchal G. A comparative study on validation of a novel cooled-wet electrode for radiofrequency liver ablation. Invest Radiol. 2000. 35:438–444.22. Boehm T, Malich A, Reichenbach JR, Fleck M, Kaiser WA. Percutaneous radiofrequency (RF) thermal ablation of rabbit tumors embedded in fat: a model for RF ablation of breast tumors. Invest Radiol. 2001. 36:480–486.23. Boehm T, Malich A, Goldberg SN, et al. Radiofrequency tumor ablation: internally cooled electrode versus saline-enhanced technique in an aggressive rabbit tumor model. Radiology. 2002. 222:805–813.24. Lee JD, Lee JM, Kim SW, Kim CS, Mun WS. MR imaging-histopathologic correlation of radiofrequency thermal ablation lesion in a rabbit liver model: observation during acute and chronic stages. Korean J Radiol. 2001. 2:151–158.25. Haaga JR. Haaga JR, Lanzieri CF, editors. Interventional CT-guided procedures. Computed tomography and magnetic resonance imaging of the whole body. 1994. 3rd ed. St.Louis: Mosby Yearbook;1572–1693.26. Neeleman N, Andersson R. Repeated liver resection for recurrent liver cancer. Br J Surg. 1996. 83:893–901.27. Cady B, Jenkins RL, Steele GD Jr, et al. Surgical margin in hepatic resection for colorectal metastases: a critical and improvable determinant of outcome. Ann Surg. 1998. 227:566–571.28. Elias D, Cavalcanti A, Sabourin JC, et al. Resection of liver metastases from colorectal cancer: the real impact of the surgical margin. Eur J Surg Oncol. 1998. 24:174–179.29. McGahan JP, Dodd GD III. Radiofrequency ablation of the liver: current status. AJR Am J Roentgenol. 2001. 176:3–16.30. Kainumao O, Asano T, Aoyama H, et al. Combined therapy with radiofrequency thermal ablation and intra-arterial infusion chemotherapy for hepatic metastases from colorectal cancer. Hepatogastroenterology. 1999. 46:1071–1077.31. Goldberg SN, Stein M, Gazelle GS, Sheiman RG, Kruskal JB, Clouse ME. Percutaneous radiofrequency tissue ablation: optimization of pulsed-RF technique to increase coagulation necrosis. J Vasc Interv Radiol. 1999. 10:907–916.32. Goldberg SN. Radiofrequency tumor ablation: principles and techniques. Eur J Ultrasound. 2001. 13:129–147.33. Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. J Appl Physiol. 1948. 1:93–122.34. Nath S, Haines DE. Biophysics and pathology of catheter energy delivery systems. Prog Cardiovasc Dis. 1995. 37:185–204.35. Rhim HS, Goldberg SN, Dodd GD II, et al. Essential techniques for successful radiofrequency thermal ablation of malignant hepatic tumors. Radiographics. 2002. 21:S17–S39.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Saline-enhanced Radiofrequency Electrocoagulation in Bovine Liver
- Radiofrequency Thermal Ablation of Hepatocellular Carcinomas
- Completely Ablated Hepatocellular Carcinoma by Percutaneous Radiofrequency Thermal Ablation
- Feasibility of Saline Infusion on the Liver Surface during Radiofrequency Ablation of Subcapsular Hepatic Tumor: An Experimental Study
- Is Percutaneous Ethanol Injection Therapy Still Effective for Hepatocellular Carcinoma in the Era of Radiofrequency Ablation?