Yonsei Med J.
2008 Jun;49(3):389-399. 10.3349/ymj.2008.49.3.389.
A Multicenter, Randomized, Open-Label, Therapeutic, and Exploratory Trial to Evaluate the Tolerability and Efficacy of Platelet Glycoprotein IIb/IIIa Receptor Blocker (Clotinab(TM)) in High-Risk Patients with Percutaneous Coronary Intervention
- Affiliations
-
- 1The Heart Center of Chonnam National University Hospital, Gwangju, Korea. Jangys1212@yuhs.ac
- 2Department of Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Division of Cardiology, Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 724253
- DOI: http://doi.org/10.3349/ymj.2008.49.3.389
Abstract
- PURPOSE
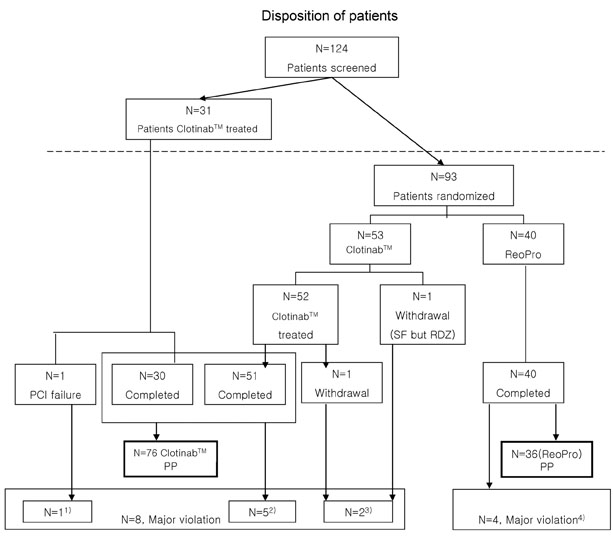
This study was designed as a multicenter, randomized, open-label study to evaluate the efficacy and tolerability of Clotinab(TM). We expected to obtain same results as with ReoPro(R) in improving ischemic cardiac complications in high-risk patients who were about to undergo percutaneous coronary intervention (PCI). PATIENTS AND METHODS: Patients of 19-80 years of age with acute coronary syndrome (ACS) who were about to undergo PCI were enrolled. After screening and confirmation of eligibility, patients were randomly assigned to different groups. Clotinab(TM) was given to 84 patients (58.7+/-10.6 years, M:F=68:16)and ReoPro(R)(59.0+/-10.5 years, M:F=30:10) was given to 40 patients before PCI. The primary efficacy endpoint was the onset of major adverse cardiac event (MACE) within 30 days from day 1. The tolerability endpoints were assessed based on bleeding, thrombocytopenia, change in Hb/Hct, human antichimetric antibody development, and adverse events. RESULTS: The number of Clotinab(TM) patients experiencing MACE was 0 out of 76 per protocol (PP) patients. The MACE rate was 0%, and its 95% exact CI was [0.00-4.74%]. A major bleeding event developed in 3 patients in the ReoPro(R) group. The probability of MACE onset in Clotinab(TM) was estimated to be less than 5%. There was no clinically significant result in tolerability variables. CONCLUSION: Clotinab(TM) is an effective and safe medicine in preventing ischemic cardiac complications for high-risk patients who will receive PCI.
Keyword
MeSH Terms
-
Acute Coronary Syndrome/surgery
Adult
Aged
Aged, 80 and over
*Angioplasty, Transluminal, Percutaneous Coronary
Antibodies, Monoclonal/adverse effects/*therapeutic use
Drugs, Investigational/adverse effects/therapeutic use
Female
Humans
Immunoglobulin Fab Fragments/adverse effects/*therapeutic use
Male
Middle Aged
Myocardial Ischemia/prevention & control
Platelet Aggregation Inhibitors/adverse effects/*therapeutic use
Platelet Glycoprotein GPIIb-IIIa Complex/*antagonists & inhibitors
Prospective Studies
Risk Factors
Treatment Outcome
Figure
Reference
-
1. Coller BS. Platelets and thrombolytic therapy. N Engl J Med. 1990. 322:33–42.
Article2. Schömig A, Neumann FJ, Kastrati A, Schühlen H, Blasini R, Hadamitzky M, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med. 1996. 334:1084–1089.
Article3. Montalescot G, Barragan P, Wittenberg O, Ecollan P, Elhadad S, Villain P, et al. Platelet glycoprotein IIb/IIIa inhibition with coronary stenting for acute myocardial infarction. N Engl J Med. 2001. 344:1895–1903.
Article4. Randomised placebo-controlled trial of abciximab before and during coronary intervention in refractory unstable angina: the CAPTURE Study. Lancet. 1997. 349:1429–1435.5. Inhibition of the platelet glycoprotein IIb/IIIa receptor with tirofiban in unstable angina and non-Q-wave myocardial infarction. Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (PRISM-PLUS) Study Investigators. N Engl J Med. 1998. 338:1488–1497.6. Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. The PURSUIT Trial Investigators. Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy. N Engl J Med. 1998. 339:436–443.7. Kong DF, Califf RM, Miller DP, Moliterno DJ, White HD, Harrington RA, et al. Clinical outcomes of therapeutic agents that block the platelet glycoprotein IIb/IIIa integrin in ischemic heart disease. Circulation. 1998. 98:2829–2835.
Article8. Coller BS. A new murine monoclonal antibody reports an activation-dependent change in the conformation and/or microenvironment of the platelet glycoprotein IIb/IIIa complex. J Clin Invest. 1985. 76:101–108.
Article9. The EPIC Investigation. Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. N Engl J Med. 1994. 330:956–961.10. Ryan TJ, Faxon DP, Gunnar RM, Kennedy JW, King SB 3rd, Loop FD, et al. Guidelines for percutaneous transluminal coronary angioplasty. A report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee on Percutaneous Transluminal Coronary Angioplasty). Circulation. 1988. 78:486–502.11. Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR, et al. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee). J Am Coll Cardiol. 2001. 38:2114–2130.
Article12. Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000. 284:835–842.
Article13. Hamm CW, Heeschen C, Goldmann B, Vahanian A, Adgey J, Miguel CM, et al. Benefit of abciximab in patients with refractory unstable angina in relation to serum troponin T levels. c7E3 Fab Antiplatelet Therapy in Unstable Refractory Angina (CAPTURE) Study Investigators. N Engl J Med. 1999. 340:1623–1629.
Article14. Simoons ML, de Boer MJ, van den Brand MJ, van Miltenburg AJ, Hoorntje JC, Heyndrickx GR, et al. Randomized trial of a GPIIb/IIIa platelet receptor blocker in refractory unstable angina. European Cooperative Study Group. Circulation. 1994. 89:596–603.
Article15. Topol EJ, Califf RM, Weisman HF, Ellis SG, Tcheng JE, Worley S, et al. Randomised trial of coronary intervention with antibody against platelet IIb/IIIa integrin for reduction of clinical restenosis: results at six months. The EPIC Investigators. Lancet. 1994. 343:881–886.
Article16. Platelet glycoprotein IIb/IIIa receptor blockade and low-dose heparin during percutaneous coronary revascularization. The EPILOG Investigators. N Engl J Med. 1997. 336:1689–1696.17. International, randomized, controlled trial of lamifiban (a platelet glycoprotein IIb/IIIa inhibitor), heparin, or both in unstable angina. The PARAGON Investigators. Platelet IIb/IIIa Antagonism for the Reduction of Acute coronary syndrome events in a Global Organization Network. Circulation. 1998. 97:2386–2395.18. A comparison of aspirin plus tirofiban with aspirin plus heparin for unstable angina. Platelet Receptor Inhibition in Ischemic Syndrome Management (PRISM) Study Investigators. N Engl J Med. 1998. 338:1498–1505.19. Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, et al. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction--summary article: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients With Unstable Angina). J Am Coll Cardiol. 2002. 40:1366–1374.20. Randomised placebo-controlled trial of effect of eptifibatide on complications of percutaneous coronary intervention: IMPACT-II. Integrilin to Minimise Platelet Aggregation and Coronary Thrombosis-II. Lancet. 1997. 349:1422–1428.21. Effects of platelet glycoprotein IIb/IIIa blockade with tirofiban on adverse cardiac events in patients with unstable angina or acute myocardial infarction undergoing coronary angioplasty. The RESTORE Investigators. Randomized Efficacy Study of Tirofiban for Outcomes and REstenosis. Circulation. 1997. 96:1445–1453.22. Alexander JH, Harrington RA. Recent antiplatelet drug trials in the acute coronary syndromes. Clinical interpretation of PRISM, PRISM-PLUS, PARAGON A and PURSUIT. Drugs. 1998. 56:965–976.
Article23. Kaul DK, Tsai HM, Liu XD, Nakada MT, Nagel RL, Coller BS. Monoclonal antibodies to alphaVbeta3 (7E3 and LM609) inhibit sickle red blood cell-endothelium interactions induced by platelet-activating factor. Blood. 2000. 95:368–374.
Article24. Thompson RD, Wakelin MW, Larbi KY, Dewar A, Asimakopoulos G, Horton MA, et al. Divergent effects of platelet-endothelial cell adhesion molecule-1 and beta 3 integrin blockade on leukocyte transmigration in vivo. J Immunol. 2000. 165:426–434.25. PRICE Investigators. Comparative 30-day economic and clinical outcomes of platelet glycoprotein IIb/IIIa inhibitor use during elective percutaneous coronary intervention: Prairie ReoPro versus Integrilin Cost Evaluation (PRICE) Trial. Am Heart J. 2001. 141:402–409.26. Coons JC, Seybert AL, Saul MI, Kirisci L, Kane-Gill SL. Outcomes and costs of abciximab versus eptifibatide for percutaneous coronary intervention. Ann Pharmacother. 2005. 39:1621–1626.27. Topol EJ, Moliterno DJ, Herrmann HC, Powers ER, Grines CL, Cohen DJ, et al. Comparison of two platelet glycoprotein IIb/IIIa inhibitors, tirofiban and abciximab, for the prevention of ischemic events with percutaneous coronary revascularization. N Engl J Med. 2001. 344:1888–1894.28. Brown DL, Fann CS, Chang CJ. Meta-analysis of effectiveness and safety of abciximab versus eptifibatide or tirofiban in percutaneous coronary intervention. Am J Cardiol. 2001. 87:537–541.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical outcome in elderly patients older than 70 years with acute myocardial infarction after use of platelet glycoprotein IIb/IIIa receptor blocker during percutaneous coronary intervention: Comparison with those younger than 70 years
- Antiplatelet Agents in High-Risk Patients with Coronary Artery Disease
- Acute Cerebral Infarction Following Intravenous Glycoprotein IIb/IIIa Inhibitor for Acute Myocardial Infarction
- The Rescue Use of A Platelet Glycoprotein IIb/IIIa Receptor Blocker (Abciximab; Reo-Pro ) in High-Risk Patients with Acute Myocardial Infarction Underwent Percutaneous Coronary Intervention
- Clinical Effects of Platelet Glycoprotein IIb/IIIa Receptor Blocker in Patients with Acute Coronary Syndrome Underwent Coronary Stenting