Yonsei Med J.
2008 Jun;49(3):383-388. 10.3349/ymj.2008.49.3.383.
Comparison of Parecoxib and Proparacetamol in Endoscopic Nasal Surgery Patients
- Affiliations
-
- 1Department of Anaesthesia and Intensive Care, Santa Maria degli Angeli Hospital, Via Montereale 24, 33170 Pordenone, Italy. yigal.leykin@aopn.fvg.it
- 2Department of Anaesthesiology, University of Parma, 43100 Parma, Italy.
- 3Department of Anaesthesia and Intensive Care, Santa Maria degli Angeli Hospital, Via Montereale 24, 33170 Pordenone, Italy.
- 4Department of ENT, Santa Maria degli Angeli Hospital, Via Montereale 24, 33170 Pordenone, Italy.
- 5Department of Anaesthesiology, University of Parma, 43100 Parma, Italy.
- KMID: 724252
- DOI: http://doi.org/10.3349/ymj.2008.49.3.383
Abstract
- PURPOSE
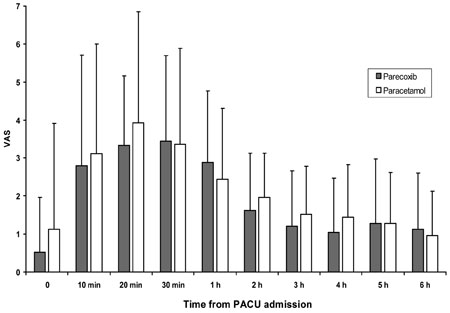
The aim of the study was to compare the efficacy of parecoxib for postoperative analgesia after endoscopic turbinate and sinus surgery with the prodrug of acetaminophen, proparacetamol. MATERIALS AND METHODS: Fifty American Society of Anesthesiology (ASA) physical status I-II patients, receiving functional endoscopic sinus surgery (FESS) and endoscopic turbinectomy, were investigated in a prospective, randomized, double-blind manner. After local infiltration with 1% mepivacaine, patients were randomly allocated to receive intravenous (IV) administration of either 40mg of parecoxib (n=25) or 2g of proparacetamol (n=25) 15 min before discontinuation of total IV anaesthesia with propofol and remifentanil. A blinded observer recorded the incidence and severity of pain at admission to the post anaesthesia care unit (PACU) at 10, 20, and 30 min after PACU admission, and every 1 h thereafter for the first 6 postoperative h. RESULTS: The area under the curve of VAS (AUC(VAS)) calculated during the study period was 669 (28-1901) cm·min in the proparacetamol group and 635 (26-1413) cm·min in the parecoxib group (p=0.34). Rescue morphine analgesia was required by 14 patients (56%) in the proparacetamol group and 12 patients (48%) in the parecoxib (p> or=0.05), while mean morphine consumption was 5-3.5mg and 5-2.0mg in the proparacetamol groups and parecoxib, respectively (p> or=0.05). No differences in the incidence of side effects were recorded between the 2 groups. Patient satisfaction was similarly high in both groups, and all patients were uneventfully discharged 24h after surgery. CONCLUSION: In patients undergoing endoscopic nasal surgery, prior infiltration with local anaesthetics, parecoxib administered before discontinuing general anaesthetic, is not superior to proparacetamol in treating early postoperative pain.
Keyword
MeSH Terms
-
Acetaminophen/administration & dosage/analogs & derivatives/*therapeutic use
Adult
Analgesics, Non-Narcotic/administration & dosage/therapeutic use
Cyclooxygenase Inhibitors/administration & dosage/therapeutic use
Double-Blind Method
Endoscopy/methods
Female
Humans
Infusions, Intravenous
Injections, Intravenous
Isoxazoles/administration & dosage/*therapeutic use
Male
Middle Aged
Nasal Polyps/surgery
Pain, Postoperative/*drug therapy
Prodrugs/administration & dosage/*therapeutic use
Prospective Studies
Sinusitis/surgery
Treatment Outcome
Figure
Reference
-
1. Slack R, Bates G. Functional endoscopic sinus surgery. Am Fam Physician. 1998. 58:707–718.2. May M, Schaitkin BM. Erasorama surgery. Curr Opin Otolaryngol Head Neck Surg. 2002. 10:19–21.
Article3. Friedman M, Venkatesan TK, Lang D, Caldarelli DD. Bupivacaine for postoperative analgesia following endoscopic sinus surgery. Laryngoscope. 1996. 106:1382–1385.
Article4. Dolin SJ, Cashman JN, Bland JM. Effectiveness of acute postoperative pain management: I. Evidence from published data. Br J Anaesth. 2002. 89:409–423.5. Church CA, Stewart C 4th, O-Lee TJ, Wallace D. Rofecoxib versus hydrocodone/acetaminophen for postoperative analgesia in functional endoscopic sinus surgery. Laryngoscope. 2006. 116:602–606.
Article6. Rømsing J, Møiniche S, Dahl JB. Rectal and parenteral paracetamol, and paracetamol in combination with NSAIDs, for postoperative analgesia. Br J Anaesth. 2002. 88:215–226.
Article7. Cheer SM, Goa KL. Parecoxib (parecoxib sodium). Drugs. 2001. 61:1133–1143.
Article8. Dalpiaz AS, Peterson D. Parecoxib: a shift in pain management? Expert Rev Neurother. 2004. 4:165–177.
Article9. Dannhardt G, Kiefer W. Cyclooxygenase inhibitors-current status and future prospects. Eur J Med Chem. 2001. 36:109–126.10. Turan A, Emet S, Karamanlioğlu B, Memis D, Turan N, Pamukcu Z. Analgesic effects of rofecoxib in ear-nose-throat surgery. Anesth Analg. 2002. 95:1308–1311.11. Browner WS, Black D, Newman B, Hulley SB. Hulley SB, Cummings SR, editors. Estimating sample size and power. Designing clinical research-an epidemiologic approach. 1988. Baltimore: Williams & Wilkins;139–150.12. Buchanan MA, Dunn GR, Macdougall GM. A prospective double-blind randomized controlled trial of the effect of topical bupivacaine on post-operative pain in bilateral nasal surgery with bilateral nasal packs inserted. J Laryngol Otol. 2005. 119:284–288.
Article13. American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2004. 100:1573–1581.14. Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, et al. COX-3 a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A. 2002. 99:13926–13931.
Article15. Ng A, Temple A, Smith G, Emembolu J. Early analgesic effects of parcoxib versus ketorolac following laparoscopic sterilization: a randomized controlled trial. Br J Anaesth. 2004. 92:846–849.
Article16. Bikhazi GB, Snabes MC, Bajwa ZH, Davis DJ, LeComte D, Traylor L, et al. A clinical trial demonstrates the analgesic activity of intravenous parecoxib sodium compared with ketorolac or morphine after gynecologic surgery with laparotomy. Am J Obstet Gynecol. 2004. 191:1183–1191.
Article17. Papadima A, Lagoudianakis EE, Antonakis PT, Pattas M, Kremastinou F, Katergiannakis V, et al. Parecoxib vs. Lornoxicam in the treatment of postoperative pain after laparoscopic cholecystectomy: a prospective randomized placebo-controlled trial. Eur J Anaesthesiol. 2007. 24:154–158.
Article18. Van Aken H, Thys L, Veekman L, Buerkle H. Assessing analgesia in single and repeated administrations of propacetamol for postoperative pain: comparison with morphine after dental surgery. Anesth Analg. 2004. 98:159–165.
Article19. Peduto VA, Ballabio M, Stefanini S. Efficacy of propacetamol in the treatment of postoperative pain. Morphine-sparing effect in orthopaedic surgery. Italian Collaborative Group on Propacetamol. Acta Anaesthesiol Scand. 1998. 42:293–298.
Article20. Varrassi G, Marinangeli F, Agro F, Aloe L, De Cillis P, De Nicola A, et al. A double-blinded evaluation of propacetamol versus ketorolac in combination with patient-controlled analgesia morphine: analgesic efficacy and tolerability after gynecologic surgery. Anesth Analg. 1999. 88:611–616.
Article21. Zhou TJ, Tang J, White PF. Propacetamol versus ketorolac for treatment of acute postoperative pain after total hip or knee replacement. Anesth Analg. 2001. 92:1569–1575.
Article22. Beaussier M, Weickmans H, Paugam C, Lavazais S, Baechle JP, Goater P, et al. A randomized, double-blind comparison between parecoxib sodium and propacetamol for parenteral postoperative analgesia after inguinal hernia repair in adult patients. Anesth Analg. 2005. 100:1309–1315.
Article23. Tilleul P, Weickmans H, Sean PT, Lienhart A, Beaussier M. Cost analysis applied to postoperative analgesia regimens: a comparison between parecoxib and propacetamol. Pharm World Sci. 2007. 29:374–379.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cyclooxygenase-2 Inhibitor Parecoxib Was Disclosed as a PPAR-γ Agonist by In Silico and In Vitro Assay
- Comparison of Preoperative and Postoperative Parecoxib Administration for Pain Control Following Major Spine Surgery
- The applications of endoscopic surgery in department of otorhinolaryngology-head and neck surgery
- Myxoma of Nasal Ala in an Adult Patient
- Mucocele of the Nasal Septum: Case Report and Review of the Literature