Ann Surg Treat Res.
2024 May;106(5):263-273. 10.4174/astr.2024.106.5.263.
CTLA4 expression profiles and their association with clinical outcomes of breast cancer: a systemic review
- Affiliations
-
- 1Department of Surgery, Konkuk University School of Medicine, Seoul, Korea
- 2Research Institute of Medical Science, Konkuk University School of Medicine, Seoul, Korea
- 3Department of Surgery, Konkuk University Medical Center, Seoul, Korea
- 4Department of Surgery, Kyung Hee University School of Medicine, Seoul, Korea
- KMID: 2555723
- DOI: http://doi.org/10.4174/astr.2024.106.5.263
Abstract
- Purpose
The cytotoxic T-lymphocyte-associated protein 4 (CTLA4) is involved in the progression of various cancers, but its biological roles in breast cancer (BRCA) remain unclear. Therefore, we performed a systematic multiomic analysis to expound on the prognostic value and underlying mechanism of CTLA4 in BRCA.
Methods
We assessed the effect of CTLA4 expression on BRCA using a variety of bioinformatics platforms, including Oncomine, GEPIA, UALCAN, PrognoScan database, Kaplan-Meier plotter, and R2: Kaplan-Meier scanner.
Results
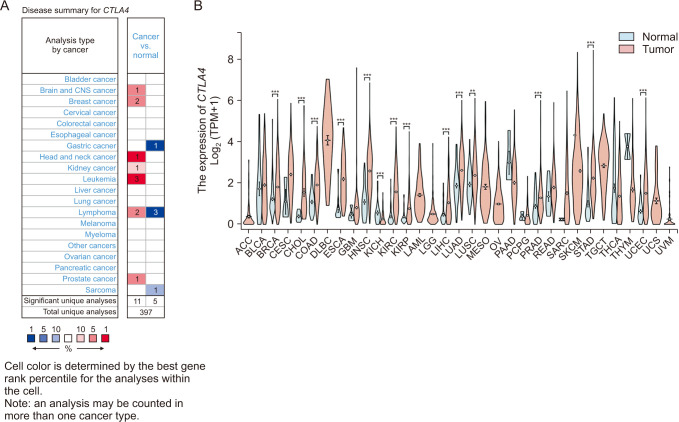
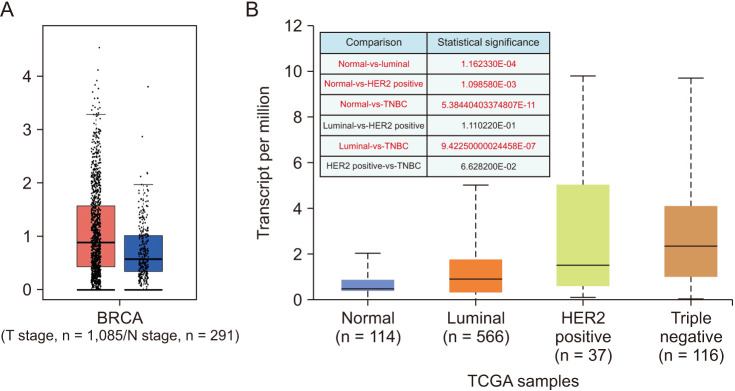
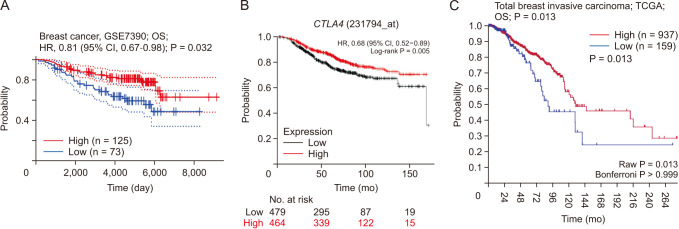
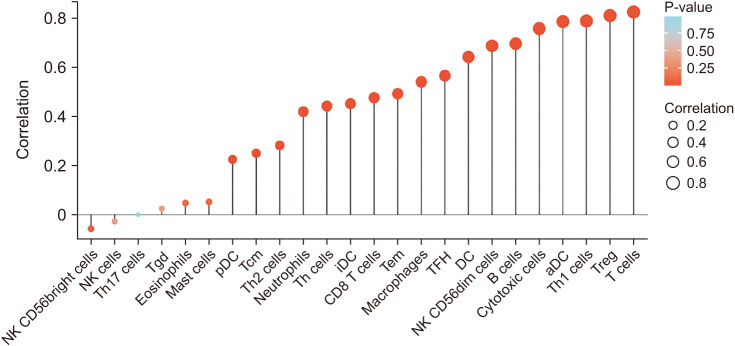
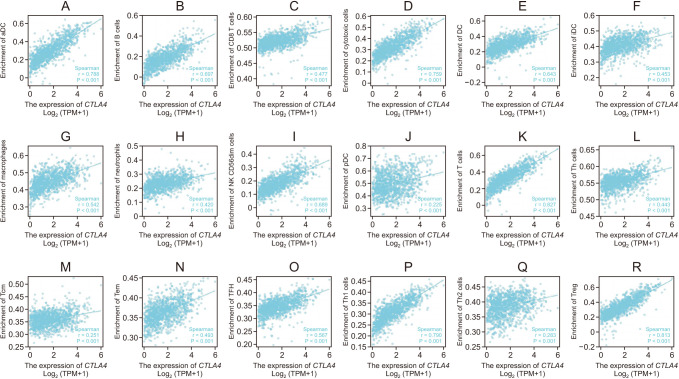
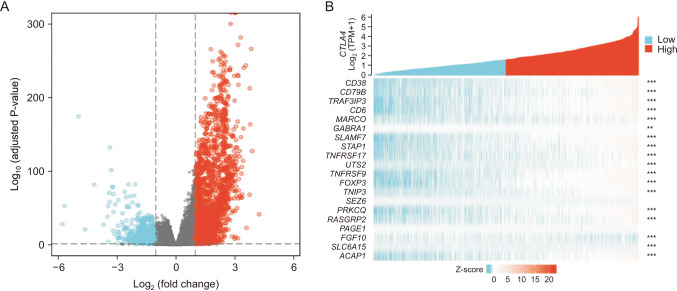
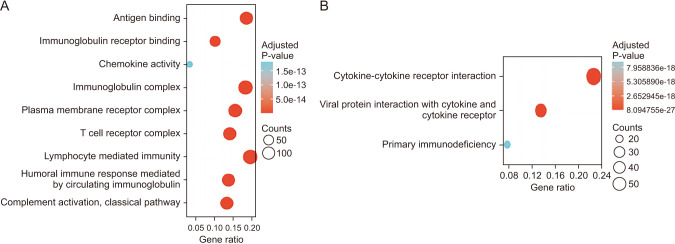
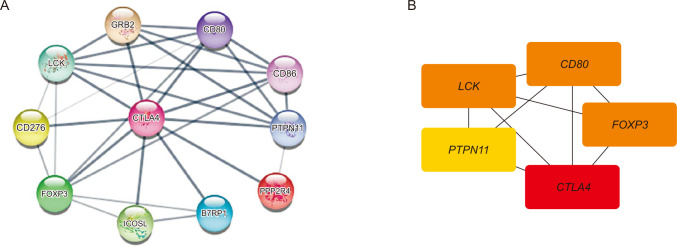
CTLA4 was highly expressed in BRCA tumor tissue compared to normal tissue (P < 0.01). The CTLA4 messenger RNA levels in BRCA based on BRCA subtypes of Luminal, human epidermal growth factor receptor 2, and triple-negative BRCA were considerably higher than in normal tissues (P < 0.001). However, the overexpression of CTLA4 was associated with a better prognosis in BRCA (P < 0.001) and was correlated with clinicopathological characteristics including age, T stage, estrogen receptors, progesterone receptors, and prediction analysis of microarray 50 (P < 0.01). The infiltration of multiple immune cells was associated with increased CTLA4 expression in BRCA (P < 0.001). CTLA4 was highly enriched in antigen binding, immunoglobulin complexes, lymphocyte-mediated immunity, and cytokine-cytokine receptor interaction.
Conclusion
This study provides suggestive evidence of the prognostic role of CTLA4 in BRCA, which may be a therapeutic target for BRCA. Furthermore, CTLA4 may influence BRCA prognosis through antigen binding, immunoglobulin complexes, lymphocyte-mediated immunity, and cytokine-cytokine receptor interaction. These findings help us understand how CTLA4 plays a role in BRCA and set the stage for more research.
Keyword
Figure
Reference
-
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–249. PMID: 33538338.2. Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019; 321:288–300. PMID: 30667505.3. Cremasco V, Astarita JL, Grauel AL, Keerthivasan S, MacIsaac K, Woodruff MC, et al. FAP delineates heterogeneous and functionally divergent stromal cells in immune-excluded breast tumors. Cancer Immunol Res. 2018; 6:1472–1485. PMID: 30266714.4. Mamessier E, Bertucci F, Sabatier R, Birnbaum D, Olive D. “Stealth” tumors: breast cancer cells shun NK-cells anti-tumor immunity. Oncoimmunology. 2012; 1:366–368. PMID: 22737617.5. Barrueto L, Caminero F, Cash L, Makris C, Lamichhane P, Deshmukh RR. Resistance to checkpoint inhibition in cancer immunotherapy. Transl Oncol. 2020; 13:100738. PMID: 32114384.6. Das M, Zhu C, Kuchroo VK. Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev. 2017; 276:97–111. PMID: 28258697.7. Yang J, He X, Lv Q, Jing J, Shi H. Management of adverse events in cancer patients treated with PD-1/PD-L1 blockade: focus on Asian populations. Front Pharmacol. 2019; 10:726. PMID: 31312140.8. Yu J, Qin B, Moyer AM, Nowsheen S, Tu X, Dong H, et al. Author Correction: regulation of sister chromatid cohesion by nuclear PD-L1. Cell Res. 2020; 30:823. PMID: 32636455.9. Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015; 348:56–61. PMID: 25838373.10. Iams WT, Porter J, Horn L. Immunotherapeutic approaches for small-cell lung cancer. Nat Rev Clin Oncol. 2020; 17:300–312. PMID: 32055013.11. Emens LA. Breast cancer immunotherapy: facts and hopes. Clin Cancer Res. 2018; 24:511–520. PMID: 28801472.12. Guan X, Wang Y, Sun Y, Zhang C, Ma S, Zhang D, et al. CTLA4-mediated immunosuppression in glioblastoma is associated with the infiltration of macrophages in the tumor microenvironment. J Inflamm Res. 2021; 14:7315–7329. PMID: 34992419.13. Bour-Jordan H, Bluestone JA. Regulating the regulators: costimulatory signals control the homeostasis and function of regulatory T cells. Immunol Rev. 2009; 229:41–66. PMID: 19426214.14. Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002; 3:611–618. PMID: 12087419.15. Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol. 2006; 18:206–213. PMID: 16464564.16. Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015; 33:1889–1894. PMID: 25667295.17. AiErken N, Shi HJ, Zhou Y, Shao N, Zhang J, Shi Y, et al. High PD-L1 expression is closely associated with tumor-infiltrating lymphocytes and leads to good clinical outcomes in Chinese triple negative breast cancer patients. Int J Biol Sci. 2017; 13:1172–1179. PMID: 29104508.18. Tomczak K, Czerwińska P, Wiznerowicz M. The Cancer Genome Atlas (TCGA): an immeasurable source of knowledge. Contemp Oncol (Pozn). 2015; 19(1A):A68–A77. PMID: 25691825.19. Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004; 6:1–6. PMID: 15068665.20. Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017; 45(W1):W98–W102. PMID: 28407145.21. Chandrashekar DS, Bashel B, Balasubramanya SA, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BV, et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017; 19:649–658. PMID: 28732212.22. Mizuno H, Kitada K, Nakai K, Sarai A. PrognoScan: a new database for meta-analysis of the prognostic value of genes. BMC Med Genomics. 2009; 2:18. PMID: 19393097.23. Lánczky A, Nagy Á, Bottai G, Munkácsy G, Szabó A, Santarpia L, et al. miRpower: a web-tool to validate survival-associated miRNAs utilizing expression data from 2178 breast cancer patients. Breast Cancer Res Treat. 2016; 160:439–446. PMID: 27744485.24. Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics. 2013; 14:7. PMID: 23323831.25. Lu T, Zheng Y, Gong X, Lv Q, Chen J, Tu Z, et al. High expression of hyaluronan-mediated motility receptor predicts adverse outcomes: a potential therapeutic target for head and neck squamous cell carcinoma. Front Oncol. 2021; 11:608842. PMID: 33763352.26. Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013; 39:782–795. PMID: 24138885.27. Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012; 16:284–287. PMID: 22455463.28. Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003; 13:2498–2504. PMID: 14597658.29. Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020; 70:313.30. Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018; 8:1069–1086. PMID: 30115704.31. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017; 377:1919–1929. PMID: 28885881.32. Powles T, O’Donnell PH, Massard C, Arkenau HT, Friedlander TW, Hoimes CJ, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol. 2017; 3:e172411. PMID: 28817753.33. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019; 380:1116–1127. PMID: 30779529.34. Bassaro L, Russell SJ, Pastwa E, Somiari SA, Somiari RI. Screening for multiple autoantibodies in plasma of patients with breast cancer. Cancer Genomics Proteomics. 2017; 14:427–435. PMID: 29109092.35. Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016; 16:275–287. PMID: 27079802.36. Cao Z, Zhang S. An integrative and comparative study of pan-cancer transcriptomes reveals distinct cancer common and specific signatures. Sci Rep. 2016; 6:33398. PMID: 27633916.37. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012; 366:2443–2454. PMID: 22658127.38. Gunturi A, McDermott DF. Potential of new therapies like anti-PD1 in kidney cancer. Curr Treat Options Oncol. 2014; 15:137–146. PMID: 24504486.39. Azoury SC, Straughan DM, Shukla V. Immune checkpoint inhibitors for cancer therapy: clinical efficacy and safety. Curr Cancer Drug Targets. 2015; 15:452–462. PMID: 26282545.40. Chen CF, Ruiz-Vega R, Vasudeva P, Espitia F, Krasieva TB, de Feraudy S, et al. ATR mutations promote the growth of melanoma tumors by modulating the immune microenvironment. Cell Rep. 2017; 18:2331–2342. PMID: 28273450.41. Liyanage UE, Law MH, Han X, An J, Ong JS, Gharahkhani P, et al. Combined analysis of keratinocyte cancers identifies novel genome-wide loci. Hum Mol Genet. 2019; 28:3148–3160. PMID: 31174203.42. Babteen NA, Fawzy MS, Alelwani W, Alharbi RA, Alruwetei AM, Toraih EA, et al. Signal peptide missense variant in cancer-brake gene CTLA4 and breast cancer outcomes. Gene. 2020; 737:144435. PMID: 32044407.43. Clarke CN, Lee MS, Wei W, Manyam G, Jiang ZQ, Lu Y, et al. Proteomic features of colorectal cancer identify tumor subtypes independent of oncogenic mutations and independently predict relapse-free survival. Ann Surg Oncol. 2017; 24:4051–4058. PMID: 28936799.44. Chakraborty G, Rangaswami H, Jain S, Kundu GC. Hypoxia regulates cross-talk between Syk and Lck leading to breast cancer progression and angiogenesis. J Biol Chem. 2006; 281:11322–11331. PMID: 16474166.45. Mahabeleshwar GH, Kundu GC. Tyrosine kinase p56lck regulates cell motility and nuclear factor kappaB-mediated secretion of urokinase type plasminogen activator through tyrosine phosphorylation of IkappaBalpha following hypoxia/reoxygenation. J Biol Chem. 2003; 278:52598–52612. PMID: 14534291.46. Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001; 27:68–73. PMID: 11138001.47. Liang YJ, Lao XM, Liang LZ, Liao GQ. Genome-wide analysis of cancer cell-derived Foxp3 target genes in human tongue squamous cell carcinoma cells. Int J Oncol. 2015; 46:1935–1943. PMID: 25779374.48. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003; 299:1057–1061. PMID: 12522256.49. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003; 4:330–336. PMID: 12612578.50. Adams TA, Vail PJ, Ruiz A, Mollaee M, McCue PA, Knudsen ES, et al. Composite analysis of immunological and metabolic markers defines novel subtypes of triple negative breast cancer. Mod Pathol. 2018; 31:288–298. PMID: 28984302.51. Chen R, Ganesan A, Okoye I, Arutyunova E, Elahi S, Lemieux MJ, et al. Targeting B7-1 in immunotherapy. Med Res Rev. 2020; 40:654–682. PMID: 31448437.52. Gazinska P, Grigoriadis A, Brown JP, Millis RR, Mera A, Gillett CE, et al. Comparison of basal-like triple-negative breast cancer defined by morphology, immunohistochemistry and transcriptional profiles. Mod Pathol. 2013; 26:955–966. PMID: 23392436.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Polymorphism of CTLA4 Gene in Schizophrenia
- A Case of Systemic Sclerosis with Coincidental Lung Cancer
- A study of expression of EGFR and ER as prognostic factors of breast cancer
- Imaging features of breast cancer molecular subtypes: state of the art
- A Case of Systemic Sclerosis Combined with Ovarian Cancer