Korean Circ J.
2024 Apr;54(4):172-186. 10.4070/kcj.2023.0197.
Dronedarone Attenuates Ang II-Induced Myocardial Hypertrophy Through Regulating SIRT1/FOXO3/PKIA Axis
- Affiliations
-
- 1Department of Cardiovascular Medicine, The First Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, China
- KMID: 2555529
- DOI: http://doi.org/10.4070/kcj.2023.0197
Abstract
- Background and Objectives
Long-term pathological myocardial hypertrophy (MH) seriously affects the normal function of the heart. Dronedarone was reported to attenuate left ventricular hypertrophy of mice. However, the molecular regulatory mechanism of dronedarone in MH is unclear.
Methods
Angiotensin II (Ang II) was used to induce cell hypertrophy of H9C2 cells. Transverse aortic constriction (TAC) surgery was performed to establish a rat model of MH. Cell size was evaluated using crystal violet staining and rhodamine phalloidin staining. Reverse transcription quantitative polymerase chain reaction and western blot were performed to detect the mRNA and protein expressions of genes. JASPAR and luciferase activity were conducted to predict and validate interaction between forkhead box O3 (FOXO3) and protein kinase inhibitor alpha (PKIA) promoter.
Results
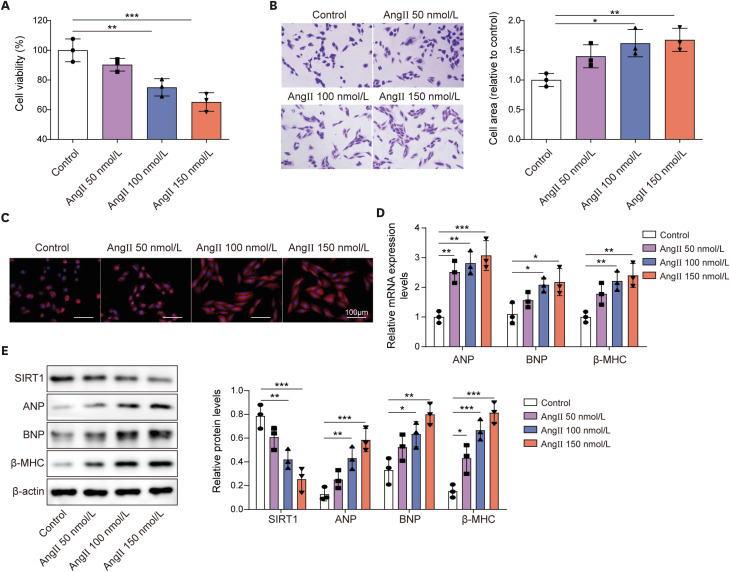
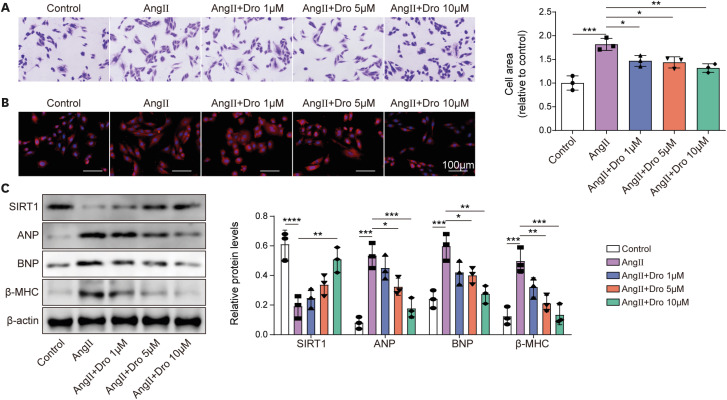
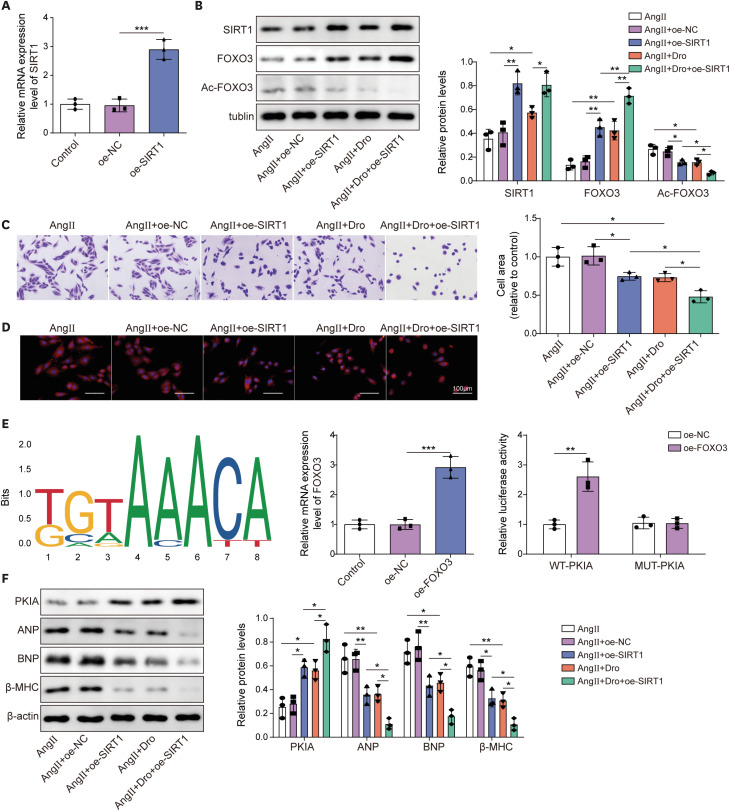
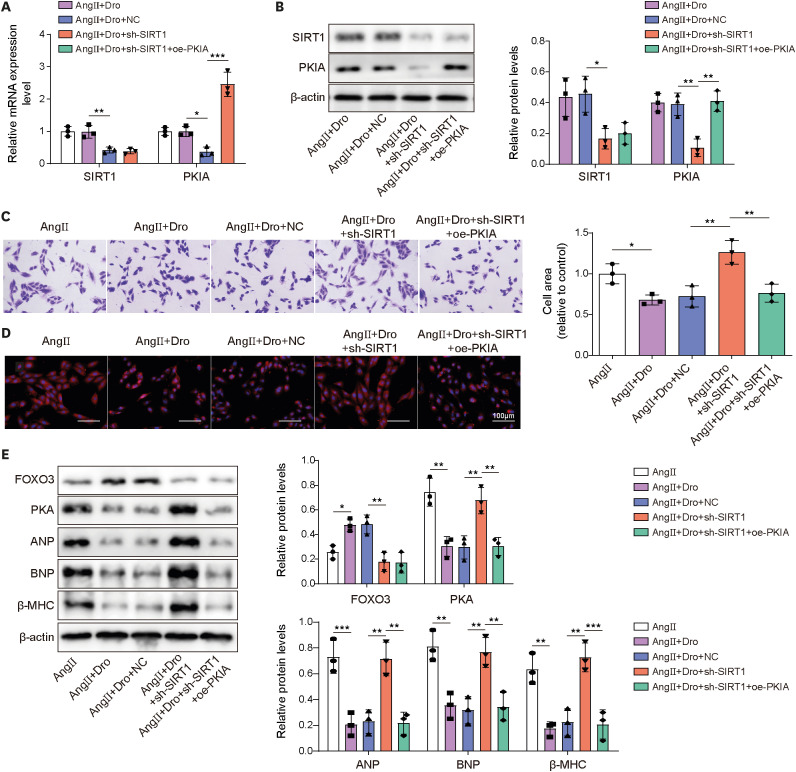
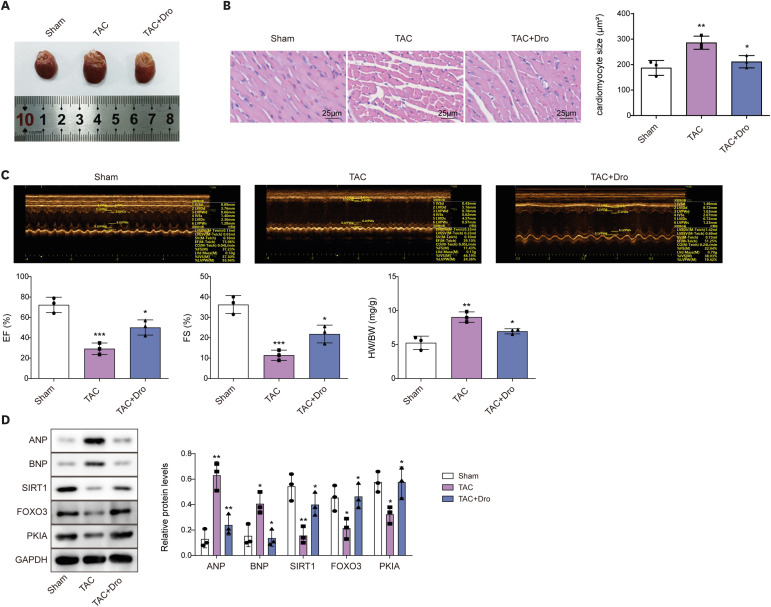
Ang II treatment induced cell hypertrophy and inhibited sirtuin 1 (SIRT1) expression, which were reversed by dronedarone. SIRT1 overexpression or PKIA overexpression enhanced dronedarone-mediated suppression of cell hypertrophy in Ang II-induced H9C2 cells. Mechanistically, SIRT1 elevated FOXO3 expression through SIRT1-mediated deacetylation of FOXO3 and FOXO3 upregulated PKIA expression through interacting with PKIA promoter. Moreover, SIRT1 silencing compromised dronedaronemediated suppression of cell hypertrophy, while PKIA upregulation abolished the influences of SIRT1 silencing. More importantly, dronedarone improved TAC surgery-induced MH and impairment of cardiac function of rats via affecting SIRT1/FOXO3/PKIA axis.
Conclusions
Dronedarone alleviated MH through mediating SIRT1/FOXO3/PKIA axis, which provide more evidences for dronedarone against MH.
Keyword
Figure
Reference
-
1. Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010; 128:191–227. PMID: 20438756.2. Shimizu I, Minamino T. Physiological and pathological cardiac hypertrophy. J Mol Cell Cardiol. 2016; 97:245–262. PMID: 27262674.3. Lei H, Hu J, Sun K, Xu D. The role and molecular mechanism of epigenetics in cardiac hypertrophy. Heart Fail Rev. 2021; 26:1505–1514. PMID: 32297065.4. Sawicka KM, Florek-Łuszczki M, Wawryniuk A, et al. Dronedarone (a multichannel blocker) enhances the anticonvulsant potency of lamotrigine, but not that of lacosamide, pregabalin and topiramate in the tonic-clonic seizure model in mice. Epilepsy Res. 2019; 154:62–68. PMID: 31059963.5. Quintana-Villamandos B, Gomez de Diego JJ, Delgado-Martos MJ, et al. Dronedarone produces early regression of myocardial remodelling in structural heart disease. PLoS One. 2017; 12:e0188442. PMID: 29161309.6. Ding RB, Bao J, Deng CX. Emerging roles of SIRT1 in fatty liver diseases. Int J Biol Sci. 2017; 13:852–867. PMID: 28808418.7. Hajializadeh Z, Khaksari M. The protective effects of 17-β estradiol and SIRT1 against cardiac hypertrophy: a review. Heart Fail Rev. 2022; 27:725–738. PMID: 34537933.8. Sundaresan NR, Pillai VB, Gupta MP. Emerging roles of SIRT1 deacetylase in regulating cardiomyocyte survival and hypertrophy. J Mol Cell Cardiol. 2011; 51:614–618. PMID: 21276800.9. Li S, Zhu Z, Xue M, et al. Fibroblast growth factor 21 protects the heart from angiotensin II-induced cardiac hypertrophy and dysfunction via SIRT1. Biochim Biophys Acta Mol Basis Dis. 2019; 1865:1241–1252. PMID: 30677512.10. Di Vincenzo S, Heijink IH, Noordhoek JA, et al. SIRT1/FoxO3 axis alteration leads to aberrant immune responses in bronchial epithelial cells. J Cell Mol Med. 2018; 22:2272–2282. PMID: 29411515.11. Yao H, Chung S, Hwang JW, et al. SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J Clin Invest. 2012; 122:2032–2045. PMID: 22546858.12. Yao J, Wang J, Xu Y, et al. CDK9 inhibition blocks the initiation of PINK1-PRKN-mediated mitophagy by regulating the SIRT1-FOXO3-BNIP3 axis and enhances the therapeutic effects involving mitochondrial dysfunction in hepatocellular carcinoma. Autophagy. 2022; 18:1879–1897. PMID: 34890308.13. Song G, Zhu L, Ruan Z, Wang R, Shen Y. MicroRNA-122 promotes cardiomyocyte hypertrophy via targeting FoxO3. Biochem Biophys Res Commun. 2019; 519:682–688. PMID: 31543343.14. Liu Y, Chen J, Fontes SK, Bautista EN, Cheng Z. Physiological and pathological roles of protein kinase A in the heart. Cardiovasc Res. 2022; 118:386–398. PMID: 33483740.15. Zhang X, Szeto C, Gao E, et al. Cardiotoxic and cardioprotective features of chronic β-adrenergic signaling. Circ Res. 2013; 112:498–509. PMID: 23104882.16. Xu J, Han Q, Shi H, Liu W, Chu T, Li H. Role of PKA in the process of neonatal cardiomyocyte hypertrophy induced by urotensin II. Int J Mol Med. 2017; 40:499–504. PMID: 28656205.17. Hoy JJ, Salinas Parra N, Park J, Kuhn S, Iglesias-Bartolome R. Protein kinase A inhibitor proteins (PKIs) divert GPCR-Gαs-cAMP signaling toward EPAC and ERK activation and are involved in tumor growth. FASEB J. 2020; 34:13900–13917. PMID: 32830375.18. Sotomayor-Flores C, Rivera-Mejías P, Vásquez-Trincado C, et al. Angiotensin-(1-9) prevents cardiomyocyte hypertrophy by controlling mitochondrial dynamics via miR-129-3p/PKIA pathway. Cell Death Differ. 2020; 27:2586–2604. PMID: 32152556.19. White PM. The role of the transcription factor Foxo3 in hearing maintenance: informed speculation on a new player in the cochlea. BioMed Res Int. 2016; 2016:1870675. PMID: 27818997.20. Ren B, Feng J, Yang N, Guo Y, Chen C, Qin Q. Ginsenoside Rg3 attenuates angiotensin II-induced myocardial hypertrophy through repressing NLRP3 inflammasome and oxidative stress via modulating SIRT1/NF-κB pathway. Int Immunopharmacol. 2021; 98:107841. PMID: 34153662.21. Vamos M, Hohnloser SH. Amiodarone and dronedarone: an update. Trends Cardiovasc Med. 2016; 26:597–602. PMID: 27155812.22. Li H, Zhou Y, Jiang B, et al. Dual effects of amiodarone on pacemaker currents in hypertrophied ventricular myocytes isolated from spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 2014; 41:698–707. PMID: 24862298.23. Takach TJ, Voigtlander JP, Jones M, Clark RE. Myocardial protective effect of amiodarone in hypertrophied hearts during global ischemia. Ann Thorac Surg. 1986; 41:542–546. PMID: 3707248.24. Zimetbaum PJ. Dronedarone for atrial fibrillation--an odyssey. N Engl J Med. 2009; 360:1811–1813. PMID: 19403901.25. Chen H, Chen X, Sun P, et al. Discovery of dronedarone and its analogues as NLRP3 inflammasome inhibitors with potent anti-inflammation activity. Bioorg Med Chem Lett. 2021; 46:128160. PMID: 34062252.26. Li Y, Ruan X, Xu X, et al. Shengmai injection suppresses angiotensin II-induced cardiomyocyte hypertrophy and apoptosis via activation of the AMPK signaling pathway through energy-dependent mechanisms. Front Pharmacol. 2019; 10:1095. PMID: 31616303.27. Cheng Y, Shen A, Wu X, et al. Qingda granule attenuates angiotensin II-induced cardiac hypertrophy and apoptosis and modulates the PI3K/AKT pathway. Biomed Pharmacother. 2021; 133:111022. PMID: 33378940.28. Zhu C, Wang M, Yu X, et al. lncRNA NBR2 attenuates angiotensin II-induced myocardial hypertrophy through repressing ER stress via activating LKB1/AMPK/Sirt1 pathway. Bioengineered. 2022; 13:13667–13679. PMID: 35703318.29. Liu Z, Li C, Yu C, Chen Z, Zhao C, Ye L. TSPYL2 reduced gefitinib resistance and DNA damage repair via suppressing SIRT1-mediated FOXO3 deacetylation. Future Med Chem. 2022; 14:407–419. PMID: 35192400.30. Senger N, C Parletta A, Marques BV, et al. Angiotensin-(1-7) prevents T3-induced cardiomyocyte hypertrophy by upregulating FOXO3/SOD1/catalase and downregulating NF-ĸB. J Cell Physiol. 2021; 236:3059–3072. PMID: 32964425.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- As a Modulator, Multitasking Roles of SIRT1 in Respiratory Diseases
- Photosensitivity caused by dronedarone: A case report
- Inhibitory effect of Hsp70 on angiotensin II-induced vascular smooth muscle cell hypertrophy
- Pitavastatin Regulates Ang II Induced Proliferation and Migration via IGFBP-5 in VSMC
- Silicate Ions Derived from Calcium Silicate Extract Decelerate Ang II-Induced Cardiac Remodeling