Korean J Pain.
2024 Apr;37(2):107-118. 10.3344/kjp.23326.
Current understanding of nociplastic pain
- Affiliations
-
- 1Department of Anesthesia and Pain Medicine, School of Medicine, Pusan National University, Yangsan, Korea
- KMID: 2554951
- DOI: http://doi.org/10.3344/kjp.23326
Abstract
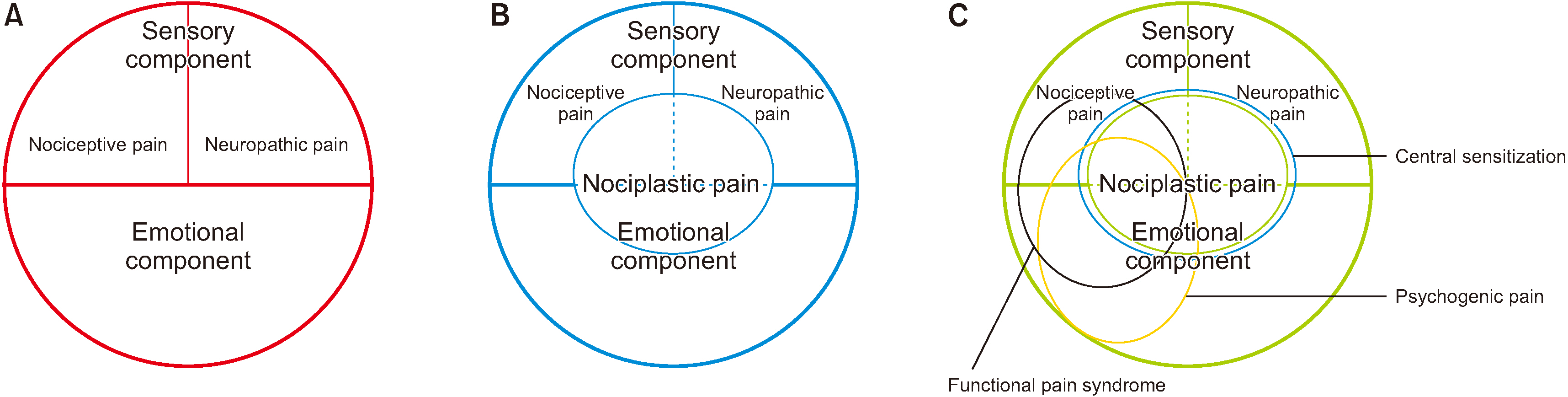
- Nociplastic pain by the “International Association for the Study of Pain” is defined as pain that arises from altered nociception despite no clear evidence of nociceptive or neuropathic pain. Augmented central nervous system pain and sensory processing with altered pain modulation are suggested to be the mechanism of nociplastic pain. Clinical criteria for possible nociplastic pain affecting somatic structures include chronic regional pain and evoked pain hypersensitivity including allodynia with after-sensation. In addition to possible nociplastic pain, clinical criteria for probable nociplastic pain are pain hypersensitivity in the region of pain to non-noxious stimuli and presence of comorbidity such as generalized symptoms with sleep disturbance, fatigue, or cognitive problems with hypersensitivity of special senses. Criteria for definitive nociplastic pain is not determined yet. Eight specific disorders related to central sensitization are suggested to be restless leg syndrome, chronic fatigue syndrome, fibromyalgia, temporomandibular disorder, migraine or tension headache, irritable bowel syndrome, multiple chemical sensitivities, and whiplash injury; non-specific emotional disorders related to central sensitization include anxiety or panic attack and depression. These central sensitization pain syndromes are overlapped to previous functional pain syndromes which are unlike organic pain syndromes and have emotional components. Therefore, nociplastic pain can be understood as chronic altered nociception related to central sensitization including both sensory components with nociceptive and/or neuropathic pain and emotional components. Nociplastic pain may be developed to explain unexplained chronic pain beyond tissue damage or pathology regardless of its origin from nociceptive, neuropathic, emotional, or mixed pain components.
Keyword
Figure
Cited by 2 articles
-
Nociplastic pain: controversy of the concept
Valdas Macionis
Korean J Pain. 2025;38(1):4-13. doi: 10.3344/kjp.24257.Nociplastic pain: conceptual and terminological considerations
Kyung Hoon Kim
Korean J Pain. 2025;38(2):87-88. doi: 10.3344/kjp.25104.
Reference
-
1. International Association for the Study of Pain (IASP). 2011. Terminology [Internet]. IASP. Available at: https://www.iasp-pain.org/resources/terminology/.2. Fitzcharles MA, Cohen SP, Clauw DJ, Littlejohn G, Usui C, Häuser W. 2021; Nociplastic pain: towards an understanding of prevalent pain conditions. Lancet. 397:2098–110. DOI: 10.1016/S0140-6736(21)00392-5. PMID: 34062144.
Article3. Kosek E, Cohen M, Baron R, Gebhart GF, Mico JA, Rice ASC, et al. 2016; Do we need a third mechanistic descriptor for chronic pain states? Pain. 157:1382–6. DOI: 10.1097/j.pain.0000000000000507. PMID: 26835783.
Article4. Nijs J, Lahousse A, Kapreli E, Bilika P, Saraçoğlu İ, Malfliet A, et al. 2021; Nociplastic pain criteria or recognition of central sensitization? Pain phenotyping in the past, present and future. J Clin Med. 10:3203. DOI: 10.3390/jcm10153203. PMID: 34361986. PMCID: PMC8347369.
Article5. Walsh DA. 2021; Nociplastic pain: helping to explain disconnect between pain and pathology. Pain. 162:2627–8. DOI: 10.1097/j.pain.0000000000002323. PMID: 34652319.
Article6. Kosek E, Clauw D, Nijs J, Baron R, Gilron I, Harris RE, et al. 2021; Chronic nociplastic pain affecting the musculoskeletal system: clinical criteria and grading system. Pain. 162:2629–34. DOI: 10.1097/j.pain.0000000000002324. PMID: 33974577.
Article7. Bułdyś K, Górnicki T, Kałka D, Szuster E, Biernikiewicz M, Markuszewski L, et al. 2023; What do we know about nociplastic pain? Healthcare (Basel). 11:1794. DOI: 10.3390/healthcare11121794. PMID: 37372912. PMCID: PMC10298569.
Article8. Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DLH, Bouhassira D, et al. 2016; Neuropathic pain: an updated grading system for research and clinical practice. Pain. 157:1599–606. DOI: 10.1097/j.pain.0000000000000492. PMID: 27115670. PMCID: PMC4949003.
Article9. Maleki J, LeBel AA, Bennett GJ, Schwartzman RJ. 2000; Patterns of spread in complex regional pain syndrome, type I (reflex sympathetic dystrophy). Pain. 88:259–66. DOI: 10.1016/S0304-3959(00)00332-8. PMID: 11068113.
Article10. van Rijn MA, Marinus J, Putter H, Bosselaar SR, Moseley GL, van Hilten JJ. 2011; Spreading of complex regional pain syndrome: not a random process. J Neural Transm (Vienna). 118:1301–9. DOI: 10.1007/s00702-011-0601-1. PMID: 21331457. PMCID: PMC3162139.
Article11. Scholz J, Finnerup NB, Attal N, Aziz Q, Baron R, Bennett MI, et al. Classification Committee of the Neuropathic Pain Special Interest Group (NeuPSIG). 2019; The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain. 160:53–9. DOI: 10.1097/j.pain.0000000000001365. PMID: 30586071. PMCID: PMC6310153.
Article12. Merskey H, Addison RG, Beric A, Blumberg H, Bogduk N, Boivie J, et al. Merskey H, Bogduk N, editors. 1994. Detailed descriptions of pain syndromes. In: Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. 2nd ed. IASP Press;p. 40–3.13. Harden NR, Bruehl S, Perez RSGM, Birklein F, Marinus J, Maihofner C, et al. 2010; Validation of proposed diagnostic criteria (the "Budapest Criteria") for complex regional pain syndrome. Pain. 150:268–74. DOI: 10.1016/j.pain.2010.04.030. PMID: 20493633. PMCID: PMC2914601.
Article14. Basch MC, Chow ET, Logan DE, Schechter NL, Simons LE. 2015; Perspectives on the clinical significance of functional pain syndromes in children. J Pain Res. 8:675–86. DOI: 10.2147/JPR.S55586. PMID: 26504406. PMCID: PMC4605245.15. Mayer EA, Bushnell MC. 2009. Functional pain syndromes: presentation and pathophysiology. 1st ed. IASP Press.16. Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. 1990; The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 33:160–72. DOI: 10.1002/art.1780330203. PMID: 2306288.17. Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. 2010; The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken). 62:600–10. DOI: 10.1002/acr.20140. PMID: 20461783.
Article18. Galvez-Sánchez CM, Reyes Del Paso GA. 2020; Diagnostic criteria for fibromyalgia: critical review and future perspectives. J Clin Med. 9:1219. DOI: 10.3390/jcm9041219. PMID: 32340369. PMCID: PMC7230253.
Article19. Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RL, et al. 2016; 2016 revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 46:319–29. DOI: 10.1016/j.semarthrit.2016.08.012. PMID: 27916278.
Article20. Arnold LM, Bennett RM, Crofford LJ, Dean LE, Clauw DJ, Goldenberg DL, et al. 2019; AAPT diagnostic criteria for fibromyalgia. J Pain. 20:611–28. DOI: 10.1016/j.jpain.2018.10.008. PMID: 30453109.
Article21. Crabtree D, Ganty P. 2016; Common functional pain syndromes. BJA Educ. 16:334–40. DOI: 10.1093/bjaed/mkw010.
Article22. National Institute for Health and Care Excellence (NICE). Irritable bowel syndrome in adult: diagnosis and management [Internet]. NICE. Available at: https://www.nice.org.uk/guidance/cg61.23. Mayer TG, Neblett R, Cohen H, Howard KJ, Choi YH, Williams MJ, et al. 2012; The development and psychometric validation of the central sensitization inventory. Pain Pract. 12:276–85. DOI: 10.1111/j.1533-2500.2011.00493.x. PMID: 21951710. PMCID: PMC3248986.
Article24. Nijs J, Torres-Cueco R, van Wilgen CP, Girbes EL, Struyf F, Roussel N, et al. 2014; Applying modern pain neuroscience in clinical practice: criteria for the classification of central sensitization pain. Pain Physician. 17:447–57. DOI: 10.36076/ppj.2014/17/447. PMID: 25247901.25. Nijs J, Malfliet A, Nishigami T. 2023; Nociplastic pain and central sensitization in patients with chronic pain conditions: a terminology update for clinicians. Braz J Phys Ther. 27:100518. DOI: 10.1016/j.bjpt.2023.100518. PMID: 37348359. PMCID: PMC10314229.
Article26. Lim LE. 1994; Psychogenic pain. Singapore Med J. 35:519–22.27. Isagulyan ED, Makashova ES, Myasnikova LK, Sergeenko EV, Aslakhanova KS, Tomskiy AA, et al. 2022; Psychogenic (nociplastic) pain: current state of diagnosis, treatment options, and potentials of neurosurgical management. Prog Brain Res. 272:105–23. DOI: 10.1016/bs.pbr.2022.03.008. PMID: 35667797.
Article