Cancer Res Treat.
2024 Apr;56(2):652-664. 10.4143/crt.2023.865.
Long-term Outcomes of Protocol-Based Treatment for Newly Diagnosed Medulloblastoma
- Affiliations
-
- 1Division of Pediatric Hematology and Oncology, Department of Pediatrics, Yonsei University Health System, Yonsei University College of Medicine, Seoul,
- 2Department of Pediatric Hemato-Oncology, Yonsei Cancer Center, Yonsei University Health System, Seoul, Korea
- 3Department of Radiation Oncology, Yonsei Cancer Center, Yonsei University Health System, Seoul, Korea
- 4Department of Radiation Oncology, Inha University Hospital, Incheon, Korea
- 5Department of Neurosurgery, Yonsei University Health System, Yonsei University College of Medicine, Seoul,
- 6Department of Radiation Oncology, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea
- 7Department of Pathology, Yonsei University Health System, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2554354
- DOI: http://doi.org/10.4143/crt.2023.865
Abstract
- Purpose
The Korean Society of Pediatric Neuro-Oncology (KSPNO) conducted treatment strategies for children with medulloblastoma (MB) by using alkylating agents for maintenance chemotherapy or tandem high-dose chemotherapy (HDC) with autologous stem cell rescue (ASCR) according to the risk stratification. The purpose of the study was to assess treatment outcomes and complications based on risk-adapted treatment and HDC.
Materials and Methods
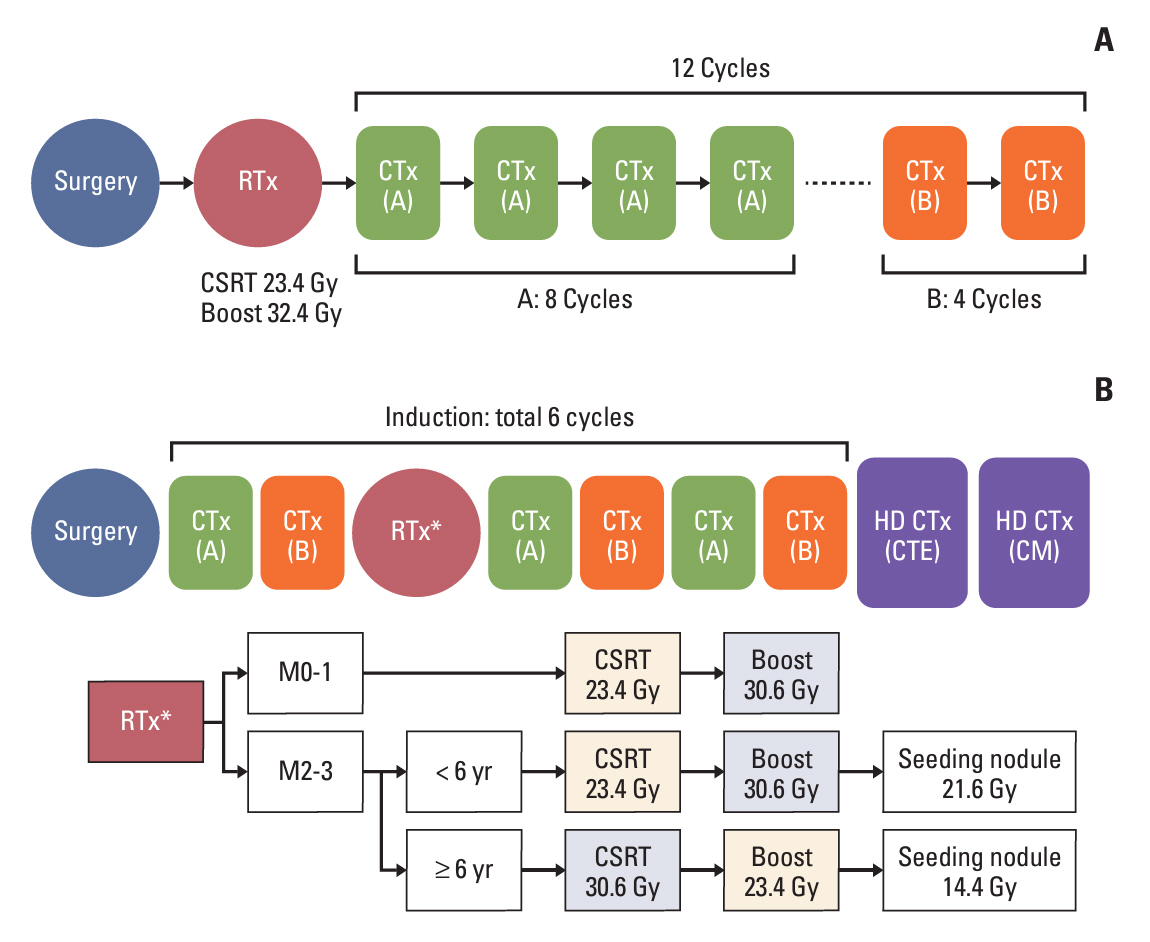
Fifty-nine patients diagnosed with MB were enrolled in this study. Patients in the standard-risk (SR) group received radiotherapy (RT) after surgery and chemotherapy using the KSPNO M051 regimen. Patients in the high-risk (HR) group received two and four chemotherapy cycles according to the KSPNO S081 protocol before and after reduced RT for age following surgery and two cycles of tandem HDC with ASCR consolidation treatment.
Results
In the SR group, 24 patients showed 5-year event-free survival (EFS) and overall survival (OS) estimates of 86.7% (95% confidence interval [CI], 73.6 to 100) and 95.8% (95% CI, 88.2 to 100), respectively. In the HR group, more infectious complications and mortality occurred during the second HDC than during the first. In the HR group, the 5-year EFS and OS estimates were 65.5% (95% CI, 51.4 to 83.4) and 72.3% (95% CI, 58.4 to 89.6), respectively.
Conclusion
High intensity of alkylating agents for SR resulted in similar outcomes but with a high incidence of hematologic toxicity. Tandem HDC with ASCR for HR induced favorable EFS and OS estimates compared to those reported previously. However, infectious complications and treatment-related mortalities suggest that a reduced chemotherapy dose is necessary, especially for the second HDC.
Figure
Reference
-
References
1. Kaatsch P, Rickert CH, Kuhl J, Schuz J, Michaelis J. Population-based epidemiologic data on brain tumors in German children. Cancer. 2001; 92:3155–64.
Article2. Ostrom QT, Price M, Ryan K, Edelson J, Neff C, Cioffi G, et al. CBTRUS statistical report: pediatric brain tumor foundation childhood and adolescent primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018. Neuro Oncol. 2022; 24:iii1–38.
Article3. Tarbell NJ, Friedman H, Polkinghorn WR, Yock T, Zhou T, Chen Z, et al. High-risk medulloblastoma: a pediatric oncology group randomized trial of chemotherapy before or after radiation therapy (POG 9031). J Clin Oncol. 2013; 31:2936–41.
Article4. Esbenshade AJ, Kocak M, Hershon L, Rousseau P, Decarie JC, Shaw S, et al. A phase II feasibility study of oral etoposide given concurrently with radiotherapy followed by dose intensive adjuvant chemotherapy for children with newly diagnosed high-risk medulloblastoma (protocol POG 9631): a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2017; 64:e26373.
Article5. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021; 23:1231–51.
Article6. Ellison D. Classifying the medulloblastoma: insights from morphology and molecular genetics. Neuropathol Appl Neurobiol. 2002; 28:257–82.
Article7. Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006; 7:813–20.
Article8. Merchant TE, Kun LE, Krasin MJ, Wallace D, Chintagumpala MM, Woo SY, et al. Multi-institution prospective trial of reduced-dose craniospinal irradiation (23.4 Gy) followed by conformal posterior fossa (36 Gy) and primary site irradiation (55.8 Gy) and dose-intensive chemotherapy for average-risk medulloblastoma. Int J Radiat Oncol Biol Phys. 2008; 70:782–7.
Article9. Zeltzer PM, Boyett JM, Finlay JL, Albright AL, Rorke LB, Milstein JM, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children’s Cancer Group 921 randomized phase III study. J Clin Oncol. 1999; 17:832–45.
Article10. Taylor RE, Bailey CC, Robinson KJ, Weston CL, Walker DA, Ellison D, et al. Outcome for patients with metastatic (M2-3) medulloblastoma treated with SIOP/UKCCSG PNET-3 chemotherapy. Eur J Cancer. 2005; 41:727–34.
Article11. Kortmann RD, Kuhl J, Timmermann B, Mittler U, Urban C, Budach V, et al. Postoperative neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood: results of the German prospective randomized trial HIT ‘91. Int J Radiat Oncol Biol Phys. 2000; 46:269–79.
Article12. Packer RJ, Goldwein J, Nicholson HS, Vezina LG, Allen JC, Ris MD, et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: a Children’s Cancer Group Study. J Clin Oncol. 1999; 17:2127–36.
Article13. Michalski JM, Janss AJ, Vezina LG, Smith KS, Billups CA, Burger PC, et al. Children’s oncology group phase III trial of reduced-dose and reduced-volume radiotherapy with chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2021; 39:2685–97.
Article14. Christopherson KM, Rotondo RL, Bradley JA, Pincus DW, Wynn TT, Fort JA, et al. Late toxicity following craniospinal radiation for early-stage medulloblastoma. Acta Oncol. 2014; 53:471–80.
Article15. Lee JW, Lim DH, Sung KW, Cho HW, Ju HY, Hyun JK, et al. Promising survival rate but high incidence of treatment-related mortality after reduced-dose craniospinal radiotherapy and tandem high-dose chemotherapy in patients with high-risk medulloblastoma. Cancer Med. 2020; 9:5807–18.
Article16. Ellison DW, Dalton J, Kocak M, Nicholson SL, Fraga C, Neale G, et al. Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol. 2011; 121:381–96.
Article17. Sung KW, Lim DH, Shin HJ. Tandem high-dose chemotherapy and autologous stem cell transplantation in children with brain tumors: review of single center experience. J Korean Neurosurg Soc. 2018; 61:393–401.
Article18. Sung KW, Lee SH, Yoo KH, Jung HL, Cho EJ, Koo HH, et al. Tandem high-dose chemotherapy and autologous stem cell rescue in patients over 1 year of age with stage 4 neuroblastoma. Bone Marrow Transplant. 2007; 40:37–45.
Article19. Packer RJ, Zhou T, Holmes E, Vezina G, Gajjar A. Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: results of Children’s Oncology Group trial A9961. Neuro Oncol. 2013; 15:97–103.
Article20. Packer RJ, Gajjar A, Vezina G, Rorke-Adams L, Burger PC, Robertson PL, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006; 24:4202–8.
Article21. Lannering B, Rutkowski S, Doz F, Pizer B, Gustafsson G, Navajas A, et al. Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: results from the randomized multicenter HIT-SIOP PNET 4 trial. J Clin Oncol. 2012; 30:3187–93.
Article22. von Bueren AO, Kortmann RD, von Hoff K, Friedrich C, Mynarek M, Muller K, et al. Treatment of children and adolescents with metastatic medulloblastoma and prognostic relevance of clinical and biologic parameters. J Clin Oncol. 2016; 34:4151–60.
Article23. Leary SE, Packer RJ, Li Y, Billups CA, Smith KS, Jaju A, et al. Efficacy of carboplatin and isotretinoin in children with high-risk medulloblastoma: a randomized clinical trial from the Children’s Oncology Group. JAMA Oncol. 2021; 7:1313–21.
Article24. Bailey S, Andre N, Gandola L, Massimino M, Rutkowski S, Clifford SC. Clinical trials in high-risk medulloblastoma: evolution of the SIOP-Europe HR-MB trial. Cancers (Basel). 2022; 14:374.
Article25. Gururangan S, Krauser J, Watral MA, Driscoll T, Larrier N, Reardon DA, et al. Efficacy of high-dose chemotherapy or standard salvage therapy in patients with recurrent medulloblastoma. Neuro Oncol. 2008; 10:745–51.
Article26. Gajjar A, Robinson GW, Smith KS, Lin T, Merchant TE, Chintagumpala M, et al. Outcomes by clinical and molecular features in children with medulloblastoma treated with risk-adapted therapy: results of an International Phase III Trial (SJMB03). J Clin Oncol. 2021; 39:822–35.
Article27. Gandola L, Massimino M, Cefalo G, Solero C, Spreafico F, Pecori E, et al. Hyperfractionated accelerated radiotherapy in the Milan strategy for metastatic medulloblastoma. J Clin Oncol. 2009; 27:566–71.
Article28. Dufour C, Foulon S, Geoffray A, Masliah-Planchon J, Figarella-Branger D, Bernier-Chastagner V, et al. Prognostic relevance of clinical and molecular risk factors in children with high-risk medulloblastoma treated in the phase II trial PNET HR+5. Neuro Oncol. 2021; 23:1163–72.
Article29. Butturini AM, Jacob M, Aguajo J, Vander-Walde NA, Villablanca J, Jubran R, et al. High-dose chemotherapy and autologous hematopoietic progenitor cell rescue in children with recurrent medulloblastoma and supratentorial primitive neuroectodermal tumors: the impact of prior radiotherapy on outcome. Cancer. 2009; 115:2956–63.
Article30. Chin AL, Moding EJ, Donaldson SS, Gibbs IC, Soltys SG, Hiniker SM, et al. Survival impact of postoperative radiotherapy timing in pediatric and adolescent medulloblastoma. Neuro Oncol. 2018; 20:1133–41.
Article31. Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012; 123:465–72.
Article32. Ramaswamy V, Taylor MD. Medulloblastoma: from myth to molecular. J Clin Oncol. 2017; 35:2355–63.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Medulloblastoma: Does the Isochromosome 17q Influence on the Long Term Survival?
- Medulloblastoma Mimicking an Extraaxial Tumor on Radiological Examination
- Molecular Pathologic Classification of Medulloblastoma
- Medulloblastoma: Current Perspectives and Recent Advances
- Isolated Supratentorial Intraventricular Recurrence of Medulloblastoma