Cancer Res Treat.
2024 Apr;56(2):580-589. 10.4143/crt.2023.812.
NESC Multicenter Phase II Trial in the Preoperative Treatment of Gastric Adenocarcinoma with Chemotherapy (Docetaxel-Cisplatin-5FU+Lenograstim) Followed by Chemoradiation Based 5FU and Oxaliplatin and Surgery
- Affiliations
-
- 1Institut Sainte Catherine, Avignon, France
- 2Centre Léon Berard, Lyon, France
- 3Hopital Saint Joseph, Paris, France
- 4INSERM, Montpellier, France
- 5Institut Paoli Calmettes, Marseille, France
- 6Hopital Henri Duffaut, Avignon, France
- 7Hopital Sud, Amiens, France
- 8CHU de Nimes, Nimes, France
- 9Hopital Saint-André, Bordeaux, France
- KMID: 2554347
- DOI: http://doi.org/10.4143/crt.2023.812
Abstract
- Purpose
Preoperative chemoradiation (CRT) is expected to increase the rate of curative resection and complete histological response. In this trial, we investigated the efficacy of a neoadjuvant CRT regimen in gastric adenocarcinoma (NCT01565109 trial).
Materials and Methods
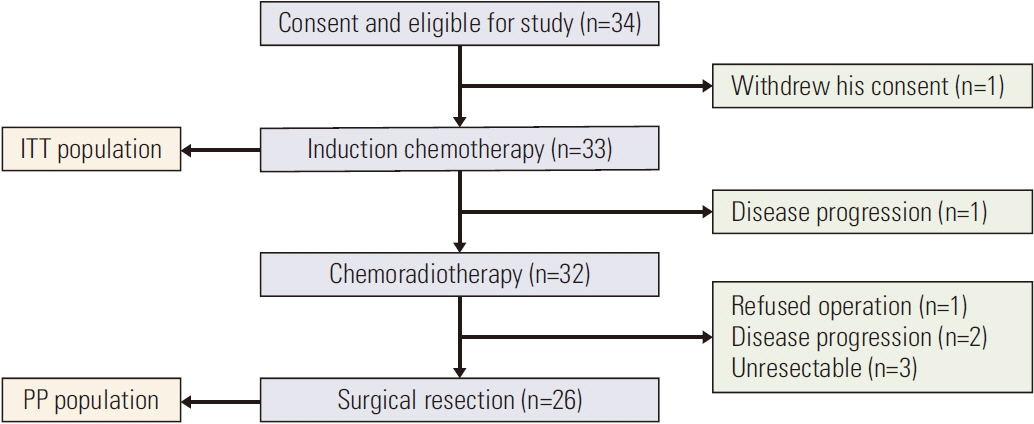
Patients with stage IB to IIIC gastric adenocarcinoma, endoscopy ultrasound and computed tomography–scan diagnosed, were eligible for this phase II trial. Neoadjuvant treatment consisted of 2 cycles of chemotherapy with DCF (docetaxel, cisplatin, and 5-fluorouracil [5FU]) followed by preoperative CRT with oxaliplatin, continuous 5FU and radiotherapy (45 Gy in 25 fractions of 1.8 Gy, 5 fractions per week for 5 weeks) administered before surgery. R0-resection rate, pathological complete response (pathCR) rate, and survival (progression-free survival [PFS] and overall survival [OS]) were evaluated as primary endpoints.
Results
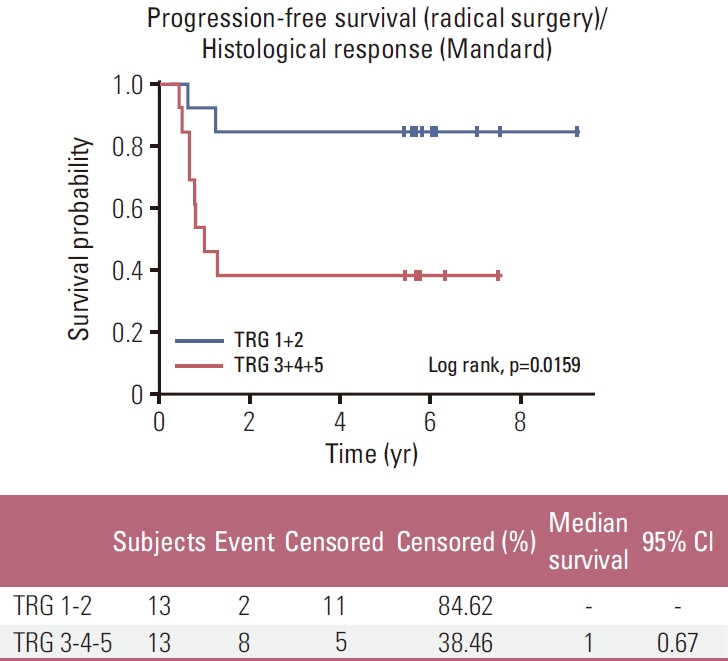
Among 33 patients included, 32 patients (97%) received CRT and 26 (78.8%) were resected (R0 resection for all patients resected). Among resected patients, we report pathCR in 23,1% and pathologic major response (tumor regression grade 2 according to Mandard’s classification) in 26,9%. With a median follow-up duration of 5.82 years (range, 0.4 to 9.24 years), the estimated median OS for all 33 patients was not reached; 1-, 3-, and 5-year OS rates were 85%, 61%, and 52%, respectively. Among resected patients, those whose histological response was tumor grade regression (TRG) 1-2 had significantly better OS and PFS rates than those with a TRG 3-4-5 response (p=0.019 and p=0.016, respectively).
Conclusion
Promising results from trials involving preoperative chemoradiation followed by surgery in gastric cancer need to be further evaluated in a phase III trial.
Figure
Reference
-
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
Article2. Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D. Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol. 2014; 40:584–91.
Article3. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006; 355:11–20.
Article4. Ychou M, Boige V, Pignon JP, Conroy T, Bouche O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011; 29:1715–21.
Article5. Jung JJ, Cho JH, Shin S, Shim YM. Surgical treatment of anastomotic recurrence after gastrectomy for gastric cancer. Korean J Thorac Cardiovasc Surg. 2014; 47:269–74.
Article6. Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: fifth edition American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis. Cancer. 2000; 88:921–32.
Article7. Ajani JA, Mansfield PF, Janjan N, Morris J, Pisters PW, Lynch PM, et al. Multi-institutional trial of preoperative chemoradiotherapy in patients with potentially resectable gastric carcinoma. J Clin Oncol. 2004; 22:2774–80.
Article8. Becker K, Langer R, Reim D, Novotny A, Meyer zum Buschenfelde C, Engel J, et al. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg. 2011; 253:934–9.9. Ajani JA, Mansfield PF, Crane CH, Wu TT, Lunagomez S, Lynch PM, et al. Paclitaxel-based chemoradiotherapy in localized gastric carcinoma: degree of pathologic response and not clinical parameters dictated patient outcome. J Clin Oncol. 2005; 23:1237–44.
Article10. Leong T, Smithers BM, Haustermans K, Michael M, Gebski V, Miller D, et al. TOPGEAR: a randomized, phase III trial of perioperative ECF chemotherapy with or without preoperative chemoradiation for resectable gastric cancer: interim results from an international, intergroup trial of the AGITG, TROG, EORTC and CCTG. Ann Surg Oncol. 2017; 24:2252–8.
Article11. Slagter AE, Jansen EPM, van Laarhoven HWM, van Sandick JW, van Grieken NCT, Sikorska K, et al. CRITICS-II: a multicentre randomised phase II trial of neo-adjuvant chemotherapy followed by surgery versus neo-adjuvant chemotherapy and subsequent chemoradiotherapy followed by surgery versus neo-adjuvant chemoradiotherapy followed by surgery in resectable gastric cancer. BMC Cancer. 2018; 18:877.
Article12. Ajani JA, Winter K, Okawara GS, Donohue JH, Pisters PW, Crane CH, et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol. 2006; 24:3953–8.
Article13. Badgwell B, Blum M, Estrella J, Chiang YJ, Das P, Matamoros A, et al. Predictors of survival in patients with resectable gastric cancer treated with preoperative chemoradiation therapy and gastrectomy. J Am Coll Surg. 2015; 221:83–90.
Article14. Kim HS, Koom WS, Baek SE, Kim HI, Jung M, Beom SH, et al. Phase II trial of preoperative sequential chemotherapy followed by chemoradiotherapy for high-risk gastric cancer. Radiother Oncol. 2019; 140:143–9.
Article15. Roth AD, Fazio N, Stupp R, Falk S, Bernhard J, Saletti P, et al. Docetaxel, cisplatin, and fluorouracil; docetaxel and cisplatin; and epirubicin, cisplatin, and fluorouracil as systemic treatment for advanced gastric carcinoma: a randomized phase II trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol. 2007; 25:3217–23.
Article16. Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006; 24:4991–7.
Article17. Al-Batran SE, Hofheinz RD, Pauligk C, Kopp HG, Haag GM, Luley KB, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016; 17:1697–708.
Article18. Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019; 393:1948–57.19. Conroy T, Galais MP, Raoul JL, Bouche O, Gourgou-Bourgade S, Douillard JY, et al. Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. Lancet Oncol. 2014; 15:305–14.
Article20. Ferri LE, Ades S, Alcindor T, Chasen M, Marcus V, Hickeson M, et al. Perioperative docetaxel, cisplatin, and 5-fluorouracil (DCF) for locally advanced esophageal and gastric adenocarcinoma: a multicenter phase II trial. Ann Oncol. 2012; 23:1512–7.
Article21. Thuss-Patience PC, Hofheinz RD, Arnold D, Florschutz A, Daum S, Kretzschmar A, et al. Perioperative chemotherapy with docetaxel, cisplatin and capecitabine (DCX) in gastrooesophageal adenocarcinoma: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO)dagger. Ann Oncol. 2012; 23:2827–34.22. Schulz C, Kullmann F, Kunzmann V, Fuchs M, Geissler M, Vehling-Kaiser U, et al. NeoFLOT: Multicenter phase II study of perioperative chemotherapy in resectable adenocarcinoma of the gastroesophageal junction or gastric adenocarcinomaVery good response predominantly in patients with intestinal type tumors. Int J Cancer. 2015; 137:678–85.
Article23. Derieux S, Svrcek M, Manela S, Lagorce-Pages C, Berger A, Andre T, et al. Evaluation of the prognostic impact of pathologic response to preoperative chemotherapy using Mandard’s tumor regression grade (TRG) in gastric adenocarcinoma. Dig Liver Dis. 2020; 52:107–14.
Article24. Rohatgi PR, Mansfield PF, Crane CH, Wu TT, Sunder PK, Ross WA, et al. Surgical pathology stage by American Joint Commission on Cancer criteria predicts patient survival after preoperative chemoradiation for localized gastric carcinoma. Cancer. 2006; 107:1475–82.
Article25. Persiani R, D’Ugo D, Rausei S, Sermoneta D, Barone C, Pozzo C, et al. Prognostic indicators in locally advanced gastric cancer (LAGC) treated with preoperative chemotherapy and D2- gastrectomy. J Surg Oncol. 2005; 89:227–36.26. Ott K, Weber WA, Lordick F, Becker K, Busch R, Herrmann K, et al. Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagogastric junction. J Clin Oncol. 2006; 24:4692–8.
Article27. Lorenzen S, Thuss-Patience P, Al-Batran SE, Lordick F, Haller B, Schuster T, et al. Impact of pathologic complete response on disease-free survival in patients with esophagogastric adenocarcinoma receiving preoperative docetaxel-based chemotherapy. Ann Oncol. 2013; 24:2068–73.
Article28. Lowy AM, Mansfield PF, Leach SD, Pazdur R, Dumas P, Ajani JA. Response to neoadjuvant chemotherapy best predicts survival after curative resection of gastric cancer. Ann Surg. 1999; 229:303–8.
Article29. Martin-Romano P, Sola JJ, Diaz-Gonzalez JA, Chopitea A, Iragorri Y, Martinez-Regueira F, et al. Role of histological regression grade after two neoadjuvant approaches with or without radiotherapy in locally advanced gastric cancer. Br J Cancer. 2016; 115:655–63.
Article30. Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010; 28:5210–8.
Article31. Cats A, Jansen EPM, van Grieken NCT, Sikorska K, Lind P, Nordsmark M, et al. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): an international, open-label, randomised phase 3 trial. Lancet Oncol. 2018; 19:616–28.32. Fuchs CS, Niedzwiecki D, Mamon HJ, Tepper JE, Ye X, Swanson RS, et al. Adjuvant chemoradiotherapy with epirubicin, cisplatin, and fluorouracil compared with adjuvant chemoradiotherapy with fluorouracil and leucovorin after curative resection of gastric cancer: results from CALGB 80101 (alliance). J Clin Oncol. 2017; 35:3671–7.
Article33. Schwartz GK, Winter K, Minsky BD, Crane C, Thomson PJ, Anne P, et al. Randomized phase II trial evaluating two paclitaxel and cisplatin-containing chemoradiation regimens as adjuvant therapy in resected gastric cancer (RTOG-0114). J Clin Oncol. 2009; 27:1956–62.
Article34. Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001; 345:725–30.
Article35. Wong RK, Jang R, Darling G. Postoperative chemoradiotherapy vs. preoperative chemoradiotherapy for locally advanced (operable) gastric cancer: clarifying the role and technique of radiotherapy. J Gastrointest Oncol. 2015; 6:89–107.36. Achilli P, De Martini P, Ceresoli M, Mari GM, Costanzi A, Maggioni D, et al. Tumor response evaluation after neoadjuvant chemotherapy in locally advanced gastric adenocarcinoma: a prospective, multi-center cohort study. J Gastrointest Oncol. 2017; 8:1018–25.
Article37. Ikoma N, Estrella JS, Blum Murphy M, Das P, Minsky BD, Mansfield P, et al. Tumor regression grade in gastric cancer after preoperative therapy. J Gastrointest Surg. 2021; 25:1380–7.
Article38. Langer R, Becker K. Tumor regression grading of gastrointestinal cancers after neoadjuvant therapy. Virchows Arch. 2018; 472:175–86.
Article39. Smyth EC, Fassan M, Cunningham D, Allum WH, Okines AF, Lampis A, et al. Effect of pathologic tumor response and nodal status on survival in the medical research council adjuvant gastric infusional chemotherapy trial. J Clin Oncol. 2016; 34:2721–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Combination chemotherapy with docetaxel and cisplatin as first-line treatment in advanced gastric cancer: is it a new effective chemotherapy?
- Chemotherapy of Advanced Gastric Cancer
- In Vitro Potentiation of 5-Fluorouracii-lnduced Cytotoxicity by Leucovorin in Human Bladder Cancer Cell Lines
- A case of peritoneal metastasis from gastric cancer successfully treated with docetaxel and cisplatin chemotherapy
- Docetaxel and cisplatin combination chemotherapy for advanced gastric cancer failed to 5-fluorouracil-based chemotherapy