J Korean Med Sci.
2024 Mar;39(10):e98. 10.3346/jkms.2024.39.e98.
Cost-Utility Analysis for Colorectal Cancer Screening According to the Initiating Age of National Cancer Screening Program in Korea
- Affiliations
-
- 1National Cancer Control Institute, National Cancer Center, Goyang, Korea
- 2Department of Health Convergence, Ewha Womans University, Seoul, Korea
- 3Graduate School of Cancer Science and Policy, National Cancer Center, Goyang, Korea
- 4Center for Colorectal Cancer, Research Institute and Hospital, National Cancer Center, Goyang, Korea
- KMID: 2554200
- DOI: http://doi.org/10.3346/jkms.2024.39.e98
Abstract
- Background
This study aimed to identify the most cost-effective strategy for colorectal cancer screening using the fecal immunochemical test (FIT), focusing on screening initiation age in Korea.
Methods
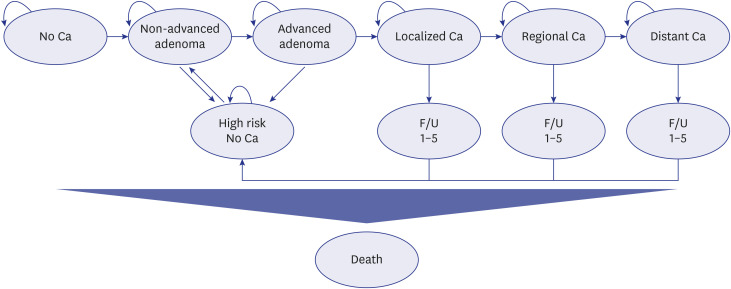
We designed Markov simulation models targeting individuals aged 40 years or older. Twelve strategies combining screening initiation ages (40, 45, or 50 years old), termination ages (80 or no limit), and intervals (1 or 2 years) were modeled, and the most cost-effective strategy was selected. The robustness of the results was confirmed using one-way and probabilistic sensitivity analyses. Furthermore, the cost-effectiveness of the qualitative and quantitative FIT methods was verified using scenario analysis.
Results
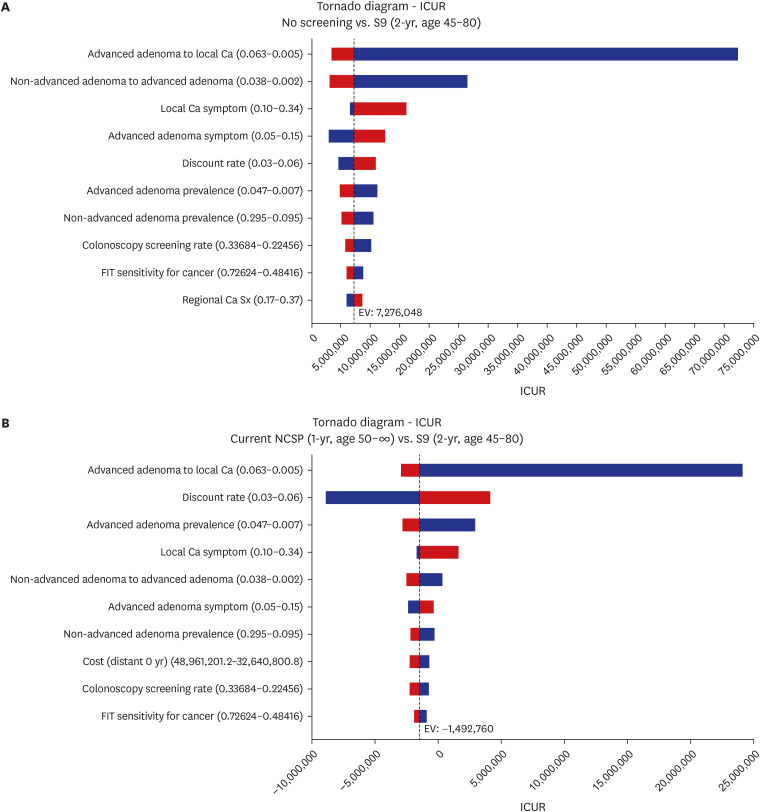
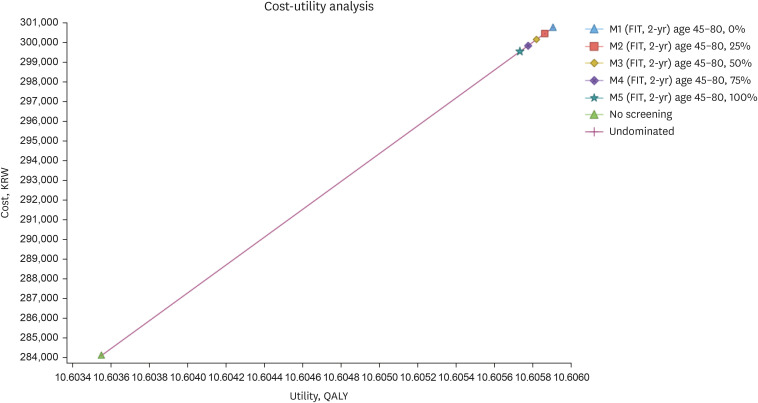
The 2-year interval strategy with a screening age range of 45–80 years was the most cost-effective (incremental cost-utility ratio = KRW 7,281,646/quality adjusted life years). The most sensitive variables in the results were transition rate from advanced adenoma to local cancer and discount rate. The uncertainty in the model was substantially low. Moreover, strategies starting at the age of 40 years were also cost-effective but considered suboptimal. The scenario analysis showed that there was no significant difference in cost-effectiveness between strategies with various relative screening ratio of quantitative and qualitative method.
Conclusion
The screening method for advancing the initiation age, as presented in the 2015 revised national screening recommendations, was superior regarding cost-effectiveness. This study provides a new paradigm for the development of a national cancer screening system in Korea, which can be utilized as a scientific basis for economic evaluations.
Figure
Reference
-
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71(3):209–249. PMID: 33538338.
Article2. Khil H, Kim SM, Hong S, Gil HM, Cheon E, Lee DH, et al. Time trends of colorectal cancer incidence and associated lifestyle factors in South Korea. Sci Rep. 2021; 11(1):2413. PMID: 33510236.
Article3. National Cancer Information Center (KR). Cancer incidence rate by age group. Updated 2020. Accessed June 2, 2023. https://www.cancer.go.kr/lay1/S1T639C642/contents.do .4. Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, et al. Colorectal cancer. Nat Rev Dis Primers. 2015; 1(1):15065. PMID: 27189416.
Article5. National Cancer Information Center (KR). Survival rate by SEER stage. Updated 2023. Accessed December 5, 2023. https://www.cancer.go.kr/lay1/S1T648C652/contents.do .6. Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, Shiratori Y. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology. 2005; 129(2):422–428. PMID: 16083699.
Article7. Ministry of Health and Welfare (KR). National Cancer Center (KR). Quality of Guidelines of Colorectal Cancer Screening: Fecal Occult Blood Test, Colonoscopy, Double Contrast Barium Enema and Pathologic Diagnosis, Secondary Revision. Goyang, Korea: National Cancer Center;2018.8. Meklin J, SyrjÄnen K, Eskelinen M. Fecal occult blood tests in colorectal cancer screening: systematic revies and meta-analysis of traditional and new-generation fecal immunochemical tests. Anticancer Res. 2020; 40(7):3591–3604. PMID: 32620599.
Article9. Siegel RL, Torre LA, Soerjomataram I, Hayes RB, Bray F, Weber TK, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019; 68(12):2179–2185. PMID: 31488504.
Article10. Sohn D, Kim M, Park Y, Suh M, Shin A, Lee H, et al. The Korean guideline for colorectal cancer screening. J Korean Med Assoc. 2015; 58(5):420–432.
Article11. Ren Y, Zhao M, Zhou D, Xing Q, Gong F, Tang W. Cost-effectiveness analysis of colonoscopy and fecal immunochemical testing for colorectal cancer screening in China. Front Public Health. 2022; 10:952378. PMID: 36033786.
Article12. Li XP, Chen HM, Lei XH, Dou GS, Chen YC, Chen LP, et al. Cost-effectiveness analysis of a community-based colorectal cancer screening program in Shanghai, China. J Dig Dis. 2021; 22(8):452–462. PMID: 34086400.
Article13. Hassan C, Benamouzig R, Spada C, Ponchon T, Zullo A, Saurin JC, et al. Cost effectiveness and projected national impact of colorectal cancer screening in France. Endoscopy. 2011; 43(9):780–793. PMID: 21623557.
Article14. Lew JB, St John DJ, Xu XM, Greuter MJ, Caruana M, Cenin DR, et al. Long-term evaluation of benefits, harms, and cost-effectiveness of the National Bowel Cancer Screening Program in Australia: a modelling study. Lancet Public Health. 2017; 2(7):e331–e340. PMID: 29253458.
Article15. Reumkens A, Rondagh EJ, Bakker CM, Winkens B, Masclee AA, Sanduleanu S. Post-colonoscopy complications: a systematic review, time trends, and meta-analysis of population-based studies. Am J Gastroenterol. 2016; 111(8):1092–1101. PMID: 27296945.
Article16. Ladabaum U, Alvarez-Osorio L, Rösch T, Brueggenjuergen B. Cost-effectiveness of colorectal cancer screening in Germany: current endoscopic and fecal testing strategies versus plasma methylated Septin 9 DNA. Endosc Int Open. 2014; 2(2):E96–104. PMID: 26135268.
Article17. Han D, Park J, Yun H, Bae S. Cost-effectiveness analysis of colon cancer screening by colonoscopic examination in Korea. Korean J Gastrointest Endosc. 2004; 28(1):1–8.18. Park S, Chang Y, Yun Y, Yoo T, Huh B, Kwon S. Cost-effectiveness analysis of colorectal cancer screening in Korean general population. J Korean Acad Fam Med. 2004; 15(25):297–306.19. Lee JY, Ock M, Jo MW, Son WS, Lee HJ, Kim SH, et al. Estimating utility weights and quality-adjusted life year loss for colorectal cancer-related health states in Korea. Sci Rep. 2017; 7(1):5571. PMID: 28717246.
Article20. Foundation for Industry Corporation, University of Ulsan. Estimation of Quality Adjusted Life Years Loss due to Major Cancers and Economic Evaluation of Cancer Screening Programs. Sejong, Korea: Ministry of Health and Welfare;2018.21. Ahn J, Kim Y, Shin S, Park J. Asian Collaborative Research Project to Determine Willingness-to-Pay per Quality-Adjusted Life Year: WTP/QALY. Seoul, Korea: National Evidence-based healthcare Collaborating Agency;2012.22. Park SM, Yun YH, Kwon S. Feasible economic strategies to improve screening compliance for colorectal cancer in Korea. World J Gastroenterol. 2005; 11(11):1587–1593. PMID: 15786532.
Article23. Park SM, Kim SY, Earle CC, Jeong SY, Yun YH. What is the most cost-effective strategy to screen for second primary colorectal cancers in male cancer survivors in Korea? World J Gastroenterol. 2009; 15(25):3153–3160. PMID: 19575496.
Article24. Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst. 1999; 91(5):434–437. PMID: 10070942.
Article25. Lieberman DA, Weiss DG. Veterans Affairs Cooperative Study Group 380. One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med. 2001; 345(8):555–560. PMID: 11529208.
Article26. Shaukat A, Mongin SJ, Geisser MS, Lederle FA, Bond JH, Mandel JS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013; 369(12):1106–1114. PMID: 24047060.
Article27. Ladabaum U, Mannalithara A, Meester RG, Gupta S, Schoen RE. Cost-effectiveness and national effects of initiating colorectal cancer screening for average-risk persons at age 45 years instead of 50 years. Gastroenterology. 2019; 157(1):137–148. PMID: 30930021.
Article28. Park MJ, Choi KS, Lee YK, Jun JK, Lee HY. A comparison of qualitative and quantitative fecal immunochemical tests in the Korean national colorectal cancer screening program. Scand J Gastroenterol. 2012; 47(4):461–466. PMID: 22428929.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cost Utility Analysis of National Cancer Screening Program for Gastric Cancer in Korea: A Markov Model Analysis
- Establishing Cancer Screening Recommendations for Major Cancers in Korea
- Screening strategy for colorectal cancer according to risk
- Colon Cancer Screening—Is It Necessary to Start under the Age of 50?
- Trends in Cancer-Screening Rates in Korea: Findings from the National Cancer Screening Survey, 2004-2023