Endocrinol Metab.
2024 Feb;39(1):164-175. 10.3803/EnM.2023.1792.
Preoperative Serum Copeptin Can Predict Delayed Hyponatremia after Pituitary Surgery in the Absence of Arginine Vasopressin Deficiency
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 2Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 3Department of Laboratory Medicine, Seoul Clinical Laboratories, Yongin, Korea
- 4Pituitary Center, Seoul National University Hospital, Seoul, Korea
- 5Department of Neurosurgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2552807
- DOI: http://doi.org/10.3803/EnM.2023.1792
Abstract
- Background
Delayed postoperative hyponatremia (DPH) is the most common cause of readmission after pituitary surgery. In this study, we aimed to evaluate the cutoff values of serum copeptin and determine the optimal timing for copeptin measurement for the prediction of the occurrence of DPH in patients who undergo endoscopic transsphenoidal approach (eTSA) surgery and tumor resection.
Methods
This was a prospective observational study of 73 patients who underwent eTSA surgery for pituitary or stalk lesions. Copeptin levels were measured before surgery, 1 hour after extubation, and on postoperative days 1, 2, 7, and 90.
Results
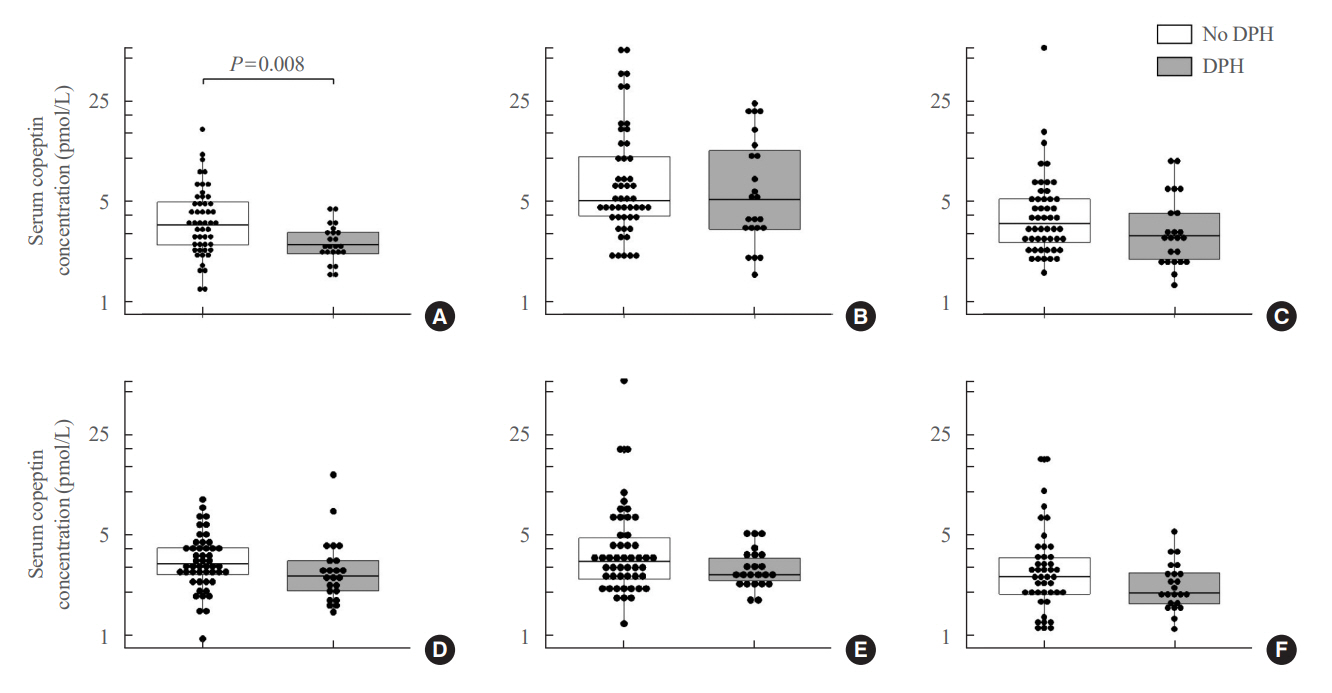
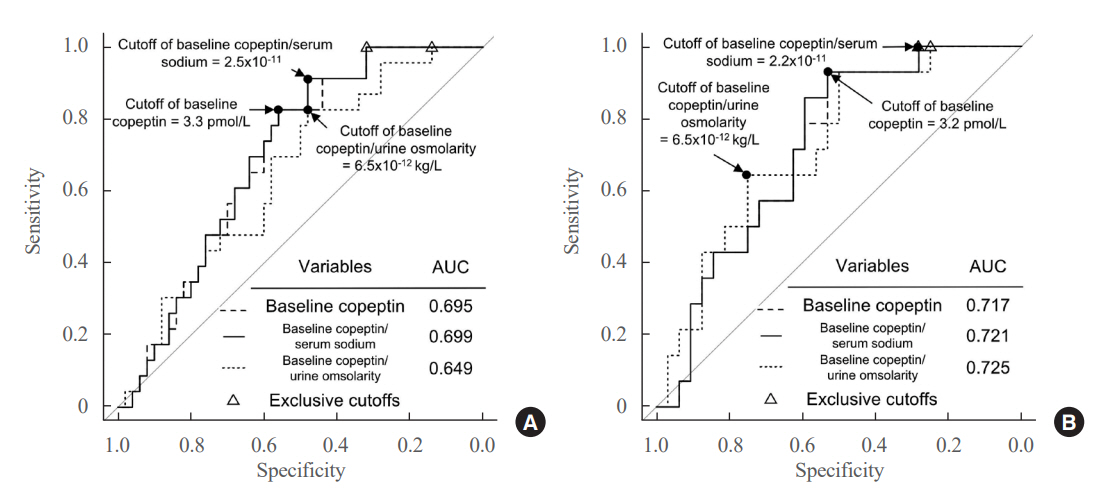
Among 73 patients, 23 patients (31.5%) developed DPH. The baseline ratio of copeptin to serum sodium level showed the highest predictive performance (area under the curve [AUROC], 0.699), and its optimal cutoff to maximize Youden’s index was 2.5×10–11, with a sensitivity of 91.3% and negative predictive value of 92.0%. No significant predictors were identified for patients with transient arginine vasopressin (AVP) deficiency. However, for patients without transient AVP deficiency, the copeptin-to-urine osmolarity ratio at baseline demonstrated the highest predictive performance (AUROC, 0.725). An optimal cutoff of 6.5×10–12 maximized Youden’s index, with a sensitivity of 92.9% and a negative predictive value of 94.1%.
Conclusion
The occurrence of DPH can be predicted using baseline copeptin and its ratio with serum sodium or urine osmolarity only in patients without transient AVP deficiency after pituitary surgery.
Figure
Reference
-
1. Bohl MA, Ahmad S, Jahnke H, Shepherd D, Knecht L, White WL, et al. Delayed hyponatremia is the most common cause of 30-day unplanned readmission after transsphenoidal surgery for pituitary tumors. Neurosurgery. 2016; 78:84–90.2. Bohl MA, Ahmad S, White WL, Little AS. Implementation of a postoperative outpatient care pathway for delayed hyponatremia following transsphenoidal surgery. Neurosurgery. 2018; 82:110–7.3. Hong YG, Kim SH, Kim EH. Delayed hyponatremia after transsphenoidal surgery for pituitary adenomas: a single institutional experience. Brain Tumor Res Treat. 2021; 9:16–20.4. Hussain NS, Piper M, Ludlam WG, Ludlam WH, Fuller CJ, Mayberg MR. Delayed postoperative hyponatremia after transsphenoidal surgery: prevalence and associated factors. J Neurosurg. 2013; 119:1453–60.5. Yoon HK, Lee HC, Kim YH, Lim YJ, Park HP. Predictive factors for delayed hyponatremia after endoscopic transsphenoidal surgery in patients with nonfunctioning pituitary tumors: a retrospective observational study. World Neurosurg. 2019; 122:e1457–64.6. Burke WT, Cote DJ, Iuliano SI, Zaidi HA, Laws ER. A practical method for prevention of readmission for symptomatic hyponatremia following transsphenoidal surgery. Pituitary. 2018; 21:25–31.7. Cote DJ, Alzarea A, Acosta MA, Hulou MM, Huang KT, Almutairi H, et al. Predictors and rates of delayed symptomatic hyponatremia after transsphenoidal surgery: a systematic review [corrected]. World Neurosurg. 2016; 88:1–6.8. Lee CC, Wang YC, Liu YT, Huang YC, Hsu PW, Wei KC, et al. Incidence and factors associated with postoperative delayed hyponatremia after transsphenoidal pituitary surgery: a meta-analysis and systematic review. Int J Endocrinol. 2021; 2021:6659152.9. Tomita Y, Kurozumi K, Inagaki K, Kameda M, Ishida J, Yasuhara T, et al. Delayed postoperative hyponatremia after endoscopic transsphenoidal surgery for pituitary adenoma. Acta Neurochir (Wien). 2019; 161:707–15.10. Deaver KE, Catel CP, Lillehei KO, Wierman ME, Kerr JM. Strategies to reduce readmissions for hyponatremia after transsphenoidal surgery for pituitary adenomas. Endocrine. 2018; 62:333–9.11. Patel KS, Shu Chen J, Yuan F, Attiah M, Wilson B, Wang MB, et al. Prediction of post-operative delayed hyponatremia after endoscopic transsphenoidal surgery. Clin Neurol Neurosurg. 2019; 182:87–91.12. Winograd D, Staggers KA, Sebastian S, Takashima M, Yoshor D, Samson SL. An effective and practical fluid restriction protocol to decrease the risk of hyponatremia and readmissions after transsphenoidal surgery. Neurosurgery. 2020; 87:761–9.13. Brooks EK, Inder WJ. Disorders of salt and water balance after pituitary surgery. J Clin Endocrinol Metab. 2022; 108:198–208.14. Lopez DC, Almeida JP, Momin AA, Andrade EJ, Soni P, Yogi-Morren D, et al. Triphasic response after endoscopic craniopharyngioma resection and its dependency on infundibular preservation or sacrifice. J Neurosurg. 2023; 139:790–7.15. Makino R, Fujio S, Hanada T, Yonenaga M, Kawade S, Hashiguchi H, et al. Delayed postoperative hyponatremia in patients with acromegaly: incidence and predictive factors. Pituitary. 2023; 26:42–50.16. Barat C, Simpson L, Breslow E. Properties of human vasopressin precursor constructs: inefficient monomer folding in the absence of copeptin as a potential contributor to diabetes insipidus. Biochemistry. 2004; 43:8191–203.17. Levy B, Chauvet MT, Chauvet J, Acher R. Ontogeny of bovine neurohypophysial hormone precursors. II. Foetal copeptin, the third domain of the vasopressin precursor. Int J Pept Protein Res. 1986; 27:320–4.18. Berton AM, Gatti F, Penner F, Varaldo E, Prencipe N, Rumbolo F, et al. Early copeptin determination allows prompt diagnosis of post-neurosurgical central diabetes insipidus. Neuroendocrinology. 2020; 110:525–34.19. Kim YH, Kim YH, Je YS, Lee KR, Lim HS, Kim JH. Changes in copeptin levels before and 3 months after transsphenoidal surgery according to the presence of postoperative central diabetes insipidus. Sci Rep. 2021; 11:17240.20. Rostom H, Noronha S, Jafar-Mohammadi B, May C, Borg A, Halliday J, et al. Post-pituitary surgery copeptin analysis as a ‘rule-out’ test for post-operative diabetes insipidus. Endocrine. 2023; 79:358–64.21. Jang HN, Kang H, Kim YH, Lim HS, Lee MK, Lee KR, et al. Serum copeptin levels at day two after pituitary surgery and ratio to baseline predict postoperative central diabetes insipidus. Pituitary. 2022; 25:1004–14.22. Knosp E, Steiner E, Kitz K, Matula C. Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery. 1993; 33:610–8.23. Kim JH, Lee JH, Lee JH, Hong AR, Kim YJ, Kim YH. Endoscopic transsphenoidal surgery outcomes in 331 nonfunctioning pituitary adenoma cases after a single surgeon learning curve. World Neurosurg. 2018; 109:e409–16.24. Dho YS, Kim YH, Se YB, Han DH, Kim JH, Park CK, et al. Endoscopic endonasal approach for craniopharyngioma: the importance of the relationship between pituitary stalk and tumor. J Neurosurg. 2018; 129:611–9.25. Loriaux DL. Diagnosis and differential diagnosis of Cushing’s syndrome. N Engl J Med. 2017; 376:1451–9.26. Wang F, Catalino MP, Bi WL, Dunn IF, Smith TR, Guo Y, et al. Postoperative day 1 morning cortisol value as a biomarker to predict long-term remission of Cushing disease. J Clin Endocrinol Metab. 2021; 106:e94–102.27. Cote DJ, Iuliano SL, Catalino MP, Laws ER. Optimizing pre-, intra-, and postoperative management of patients with sellar pathology undergoing transsphenoidal surgery. Neurosurg Focus. 2020; 48:E2.28. Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006; 52:112–9.29. Fenske W, Stork S, Blechschmidt A, Maier SG, Morgenthaler NG, Allolio B. Copeptin in the differential diagnosis of hyponatremia. J Clin Endocrinol Metab. 2009; 94:123–9.30. Christ-Crain M, Morgenthaler NG, Fenske W. Copeptin as a biomarker and a diagnostic tool in the evaluation of patients with polyuria-polydipsia and hyponatremia. Best Pract Res Clin Endocrinol Metab. 2016; 30:235–47.31. Nigro N, Winzeler B, Suter-Widmer I, Schuetz P, Arici B, Bally M, et al. Evaluation of copeptin and commonly used laboratory parameters for the differential diagnosis of profound hyponatraemia in hospitalized patients: ‘The Co-MED Study’. Clin Endocrinol (Oxf). 2017; 86:456–62.32. Baldrighi M, Castello LM, Bartoli E. Copeptin in hyponatremia: is there a role for this biomarker in the diagnostic workup? Endocrine. 2018; 60:384–5.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sick Sinus Syndrome Following Severe Hyponatremia Associated with Desmopressin Therapy
- Serum copeptin as a predictor of risk of hyponatremia after transurethral prostatectomy
- Pathophysiology of copeptin in kidney disease and hypertension
- Three Cases of Hyponatremia Caused by Ingestion of Bowel Preparation Solution for Colonoscopy
- A Case of Idiopathic Central Diabetes Insipidus together with Primary Empty Sella and Combined Pituitary Hormone Deficiency