Brain Tumor Res Treat.
2024 Jan;12(1):70-74. 10.14791/btrt.2023.0042.
Lynch Syndrome-Associated Glioblastoma Treated With Concomitant Chemoradiotherapy and Immune Checkpoint Inhibitors: Case Report and Review of Literature
- Affiliations
-
- 1Department of Neurosurgery, Nara Medical University, Kashihara, Japan
- 2Department of Diagnostic Pathology, Nara Medical University, Kashihara, Japan
- KMID: 2552343
- DOI: http://doi.org/10.14791/btrt.2023.0042
Abstract
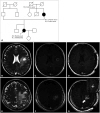
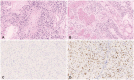
- Lynch syndrome (LS) is an autosomal dominant disorder caused by mutations in mismatch repair (MMR) genes and is also known to be associated with glioblastomas. The efficacy of immunotherapy for LS-associated glioblastomas remains unknown. Herein, we report a rare case of LS-associated glioblastoma, treated with chemotherapy using immune checkpoint inhibitors (ICI). A 41-year-old female patient presented with headaches and sensory disturbances in the right upper limb for 6 weeks. She had been treated for rectal cancer and had a family history of LS. MRI revealed two ring-enhancing lesions in the left precentral gyrus. She underwent subtotal resection, leading to a pathological diagnosis of isocitrate dehydrogenase wild-type glioblastoma. She received daily administration of (temozolomide, 75 mg/m 2 ) and concurrent radiotherapy (60 Gy) postoperatively. However, the tumor recurred 1 year after the initial treatment. A molecular genetic study showed high microsatellite instability (MSI), and she was treated with pembrolizumab therapy. Disease progression occurred despite six cycles of pembrolizumab therapy and radiotherapy at the dose of 40 Gy. She died due to glioblastoma progression 19 months after the initial treatment. The present case demonstrates that some LS-associated glioblastomas may be resistant to ICI despite high MSI, possibly because of intratumor heterogeneity related to MMR deficiency.
Keyword
Figure
Reference
-
1. Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003; 348:919–932. PMID: 12621137.
Article2. Wimmer K, Etzler J. Constitutional mismatch repair-deficiency syndrome: have we so far seen only the tip of an iceberg? Hum Genet. 2008; 124:105–122. PMID: 18709565.
Article3. Barrow E, Robinson L, Alduaij W, Shenton A, Clancy T, Lalloo F, et al. Cumulative lifetime incidence of extracolonic cancers in Lynch syndrome: a report of 121 families with proven mutations. Clin Genet. 2009; 75:141–149. PMID: 19215248.
Article4. Watson P, Vasen HFA, Mecklin JP, Bernstein I, Aarnio M, Järvinen HJ, et al. The risk of extra-colonic, extra-endometrial cancer in the Lynch syndrome. Int J Cancer. 2008; 123:444–449. PMID: 18398828.
Article5. Vasen HF, Stormorken A, Menko FH, Nagengast FM, Kleibeuker JH, Griffioen G, et al. MSH2 mutation carriers are at higher risk of cancer than MLH1 mutation carriers: a study of hereditary nonpolyposis colorectal cancer families. J Clin Oncol. 2001; 19:4074–4080. PMID: 11600610.
Article6. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017; 357:409–413. PMID: 28596308.7. Barrow E, Hill J, Evans DG. Cancer risk in Lynch syndrome. Fam Cancer. 2013; 12:229–240. PMID: 23604856.
Article8. Therkildsen C, Ladelund S, Rambech E, Persson A, Petersen A, Nilbert M. Glioblastomas, astrocytomas and oligodendrogliomas linked to Lynch syndrome. Eur J Neurol. 2015; 22:717–724. PMID: 25648859.
Article9. Moreira L, Balaguer F, Lindor N, de la Chapelle A, Hampel H, Aaltonen LA, et al. Identification of Lynch syndrome among patients with colorectal cancer. JAMA. 2012; 308:1555–1565. PMID: 23073952.
Article10. Bhattacharya P, McHugh TW. Lynch syndrome. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing;2023. Accessed October 29, 2023. at https://www.ncbi.nlm.nih.gov/books/NBK431096/.11. Kim H, Lim KY, Park JW, Kang J, Won JK, Lee K, et al. Sporadic and Lynch syndrome-associated mismatch repair-deficient brain tumors. Lab Invest. 2022; 102:160–171. PMID: 34848827.
Article12. Kawaguchi K, Otani R, Kikuchi M, Kushihara Y, Funata N, Yamada R, et al. Genetic characteristics of mismatch repair-deficient glioblastoma. NMC Case Rep J. 2021; 8:565–571. PMID: 35079518.
Article13. Kloth M, Ruesseler V, Engel C, Koenig K, Peifer M, Mariotti E, et al. Activating ERBB2/HER2 mutations indicate susceptibility to pan-HER inhibitors in Lynch and Lynch-like colorectal cancer. Gut. 2016; 65:1296–1305. PMID: 26001389.
Article14. Ahadova A, von Knebel Doeberitz M, Bläker H, Kloor M. CTNNB1-mutant colorectal carcinomas with immediate invasive growth: a model of interval cancers in Lynch syndrome. Fam Cancer. 2016; 15:579–586. PMID: 26960970.
Article15. Wang L, Ge J, Lan Y, Shi Y, Luo Y, Tan Y, et al. Tumor mutational burden is associated with poor outcomes in diffuse glioma. BMC Cancer. 2020; 20:213. PMID: 32164609.
Article16. Daniel P, Sabri S, Chaddad A, Meehan B, Jean-Claude B, Rak J, et al. Temozolomide induced hypermutation in glioma: evolutionary mechanisms and therapeutic opportunities. Front Oncol. 2019; 9:41. PMID: 30778375.
Article17. Goenka A, Tiek D, Song X, Huang T, Hu B, Cheng SY. The many facets of therapy resistance and tumor recurrence in glioblastoma. Cells. 2021; 10:484. PMID: 33668200.
Article18. Cohen R, Colle R, Pudlarz T, Heran M, Duval A, Svrcek M, et al. Immune checkpoint inhibition in metastatic colorectal cancer harboring microsatellite instability or mismatch repair deficiency. Cancers (Basel). 2021; 13:1149. PMID: 33800202.
Article19. McCord M, Steffens A, Javier R, Kam KL, McCortney K, Horbinski C. The efficacy of DNA mismatch repair enzyme immunohistochemistry as a screening test for hypermutated gliomas. Acta Neuropathol Commun. 2020; 8:15. PMID: 32051040.
Article20. Mukasa A. Genome medicine for brain tumors: current status and future perspectives. Neurol Med Chir (Tokyo). 2020; 60:531–542. PMID: 33071276.
Article21. Cahill DP, Levine KK, Betensky RA, Codd PJ, Romany CA, Reavie LB, et al. Loss of the mismatch repair protein MSH6 in human glioblastomas is associated with tumor progression during temozolomide treatment. Clin Cancer Res. 2007; 13:2038–2045. PMID: 17404084.
Article22. Touat M, Li YY, Boynton AN, Spurr LF, Iorgulescu JB, Bohrson CL, et al. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature. 2020; 580:517–523. PMID: 32322066.
Article23. Yao ZG, Hua F, Yin ZH, Xue YJ, Hou YH, Nie YC, et al. Characteristics of glioblastomas and immune microenvironment in a Chinese family with Lynch syndrome and concurrent porokeratosis. Front Oncol. 2023; 13:1194232. PMID: 37529690.
Article24. Anghileri E, Di Ianni N, Paterra R, Langella T, Zhao J, Eoli M, et al. High tumor mutational burden and T-cell activation are associated with long-term response to anti-PD1 therapy in Lynch syndrome recurrent glioblastoma patient. Cancer Immunol Immunother. 2021; 70:831–842. PMID: 33140187.
Article25. Cho YA, Kim D, Lee B, Shim JH, Suh YL. Incidence, clinicopathologic, and genetic characteristics of mismatch repair gene-mutated glioblastomas. J Neurooncol. 2021; 153:43–53. PMID: 33864561.
Article26. Hodges TR, Ott M, Xiu J, Gatalica Z, Swensen J, Zhou S, et al. Mutational burden, immune checkpoint expression, and mismatch repair in glioma: implications for immune checkpoint immunotherapy. Neuro Oncol. 2017; 19:1047–1057. PMID: 28371827.
Article27. Indraccolo S, Lombardi G, Fassan M, Pasqualini L, Giunco S, Marcato R, et al. Genetic, epigenetic, and immunologic profiling of MMR-deficient relapsed glioblastoma. Clin Cancer Res. 2019; 25:1828–1837. PMID: 30514778.
Article28. Friedman JS, Jun T, Rashidipour O, Huang KL, Ellis E, Kadaba P, et al. Using EGFR amplification to stratify recurrent glioblastoma treated with immune checkpoint inhibitors. Cancer Immunol Immunother. 2023; 72:1893–1901. PMID: 36707424.
Article29. Sherman WJ, Vitaz TW. Nivolumab with radiation therapy in a glioblastoma patient with Lynch syndrome. BMJ Case Rep. 2021; 14:e241026.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gut microbiome on immune checkpoint inhibitor therapy and consequent immune-related colitis: a review
- Red Blood Cell Autoantibodies in Patients Treated with Immune Checkpoint Inhibitors
- Treatment of advanced urogenital cancers with immune checkpoint inhibitors
- Gastrointestinal cancer treatment with immune checkpoint inhibitors
- Current status of cancer immunotherapy with immune checkpoint inhibitors