Ann Lab Med.
2023 Nov;43(6):531-538. 10.3343/alm.2023.43.6.531.
Clot Waveform Analysis for Hemostatic Abnormalities
- Affiliations
-
- 1Department of Laboratory and General Medicine, Mie Prefectural General Medical Center, Yokkaichi, Japan
- 2Department of Transfusion Medicine and Cell Therapy, Mie University Hospital, Tsu, Japan
- 3Mie Prefectural General Medical Center, Yokkaichi, Japan
- 4Department of Molecular Pathobiology and Cell Adhesion Biology, Mie University Graduate School of Medicine, Tsu, Japan
- KMID: 2552022
- DOI: http://doi.org/10.3343/alm.2023.43.6.531
Abstract
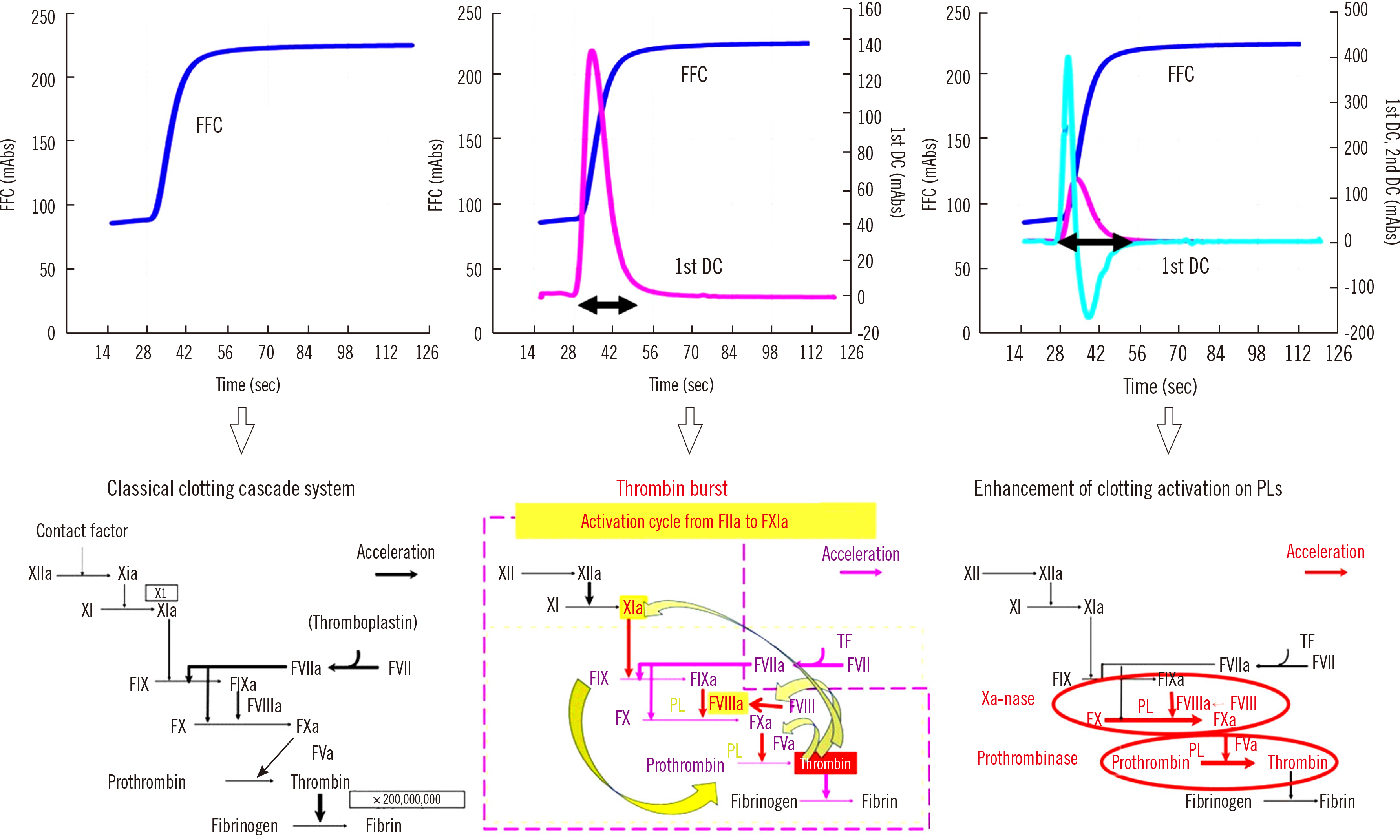
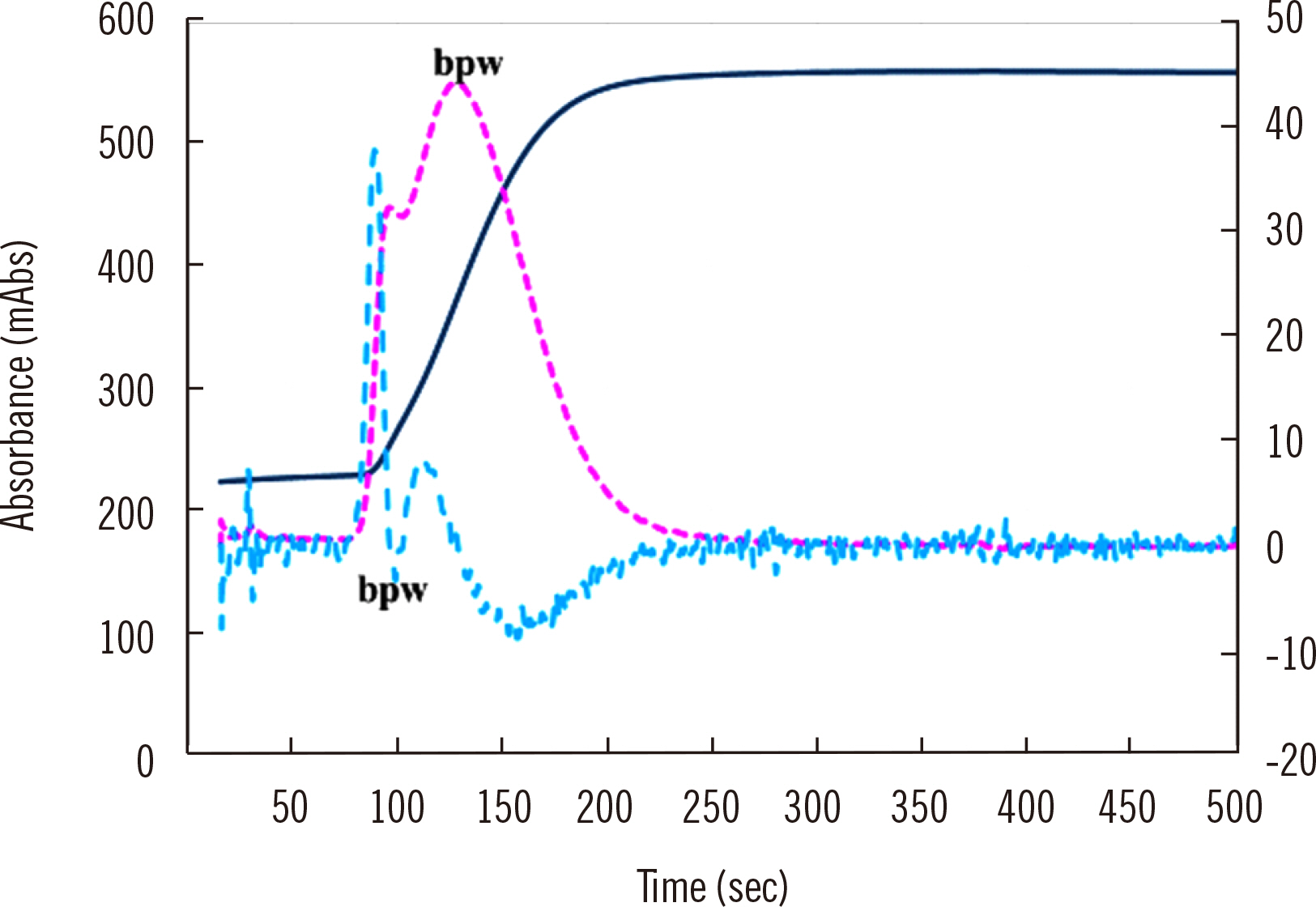
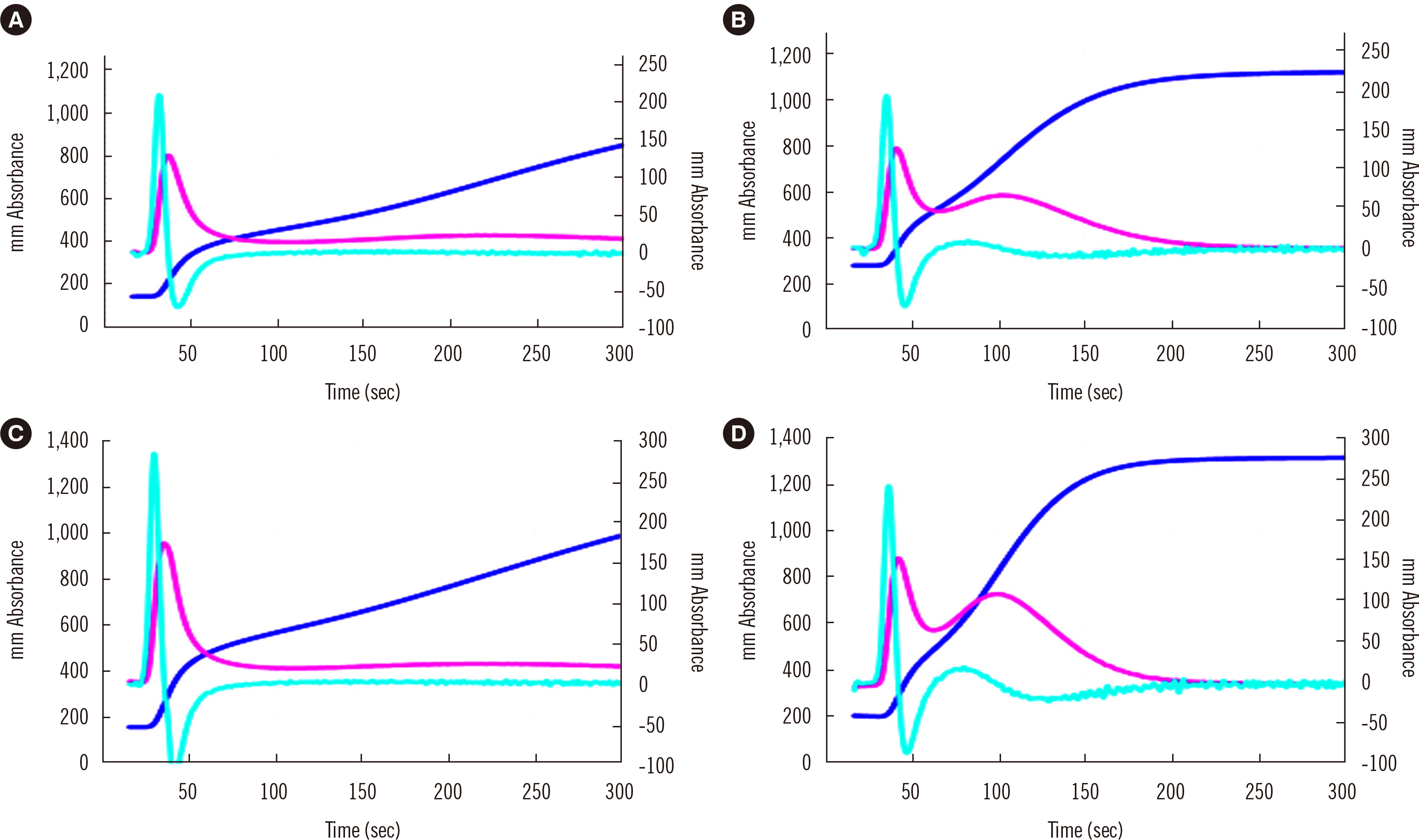
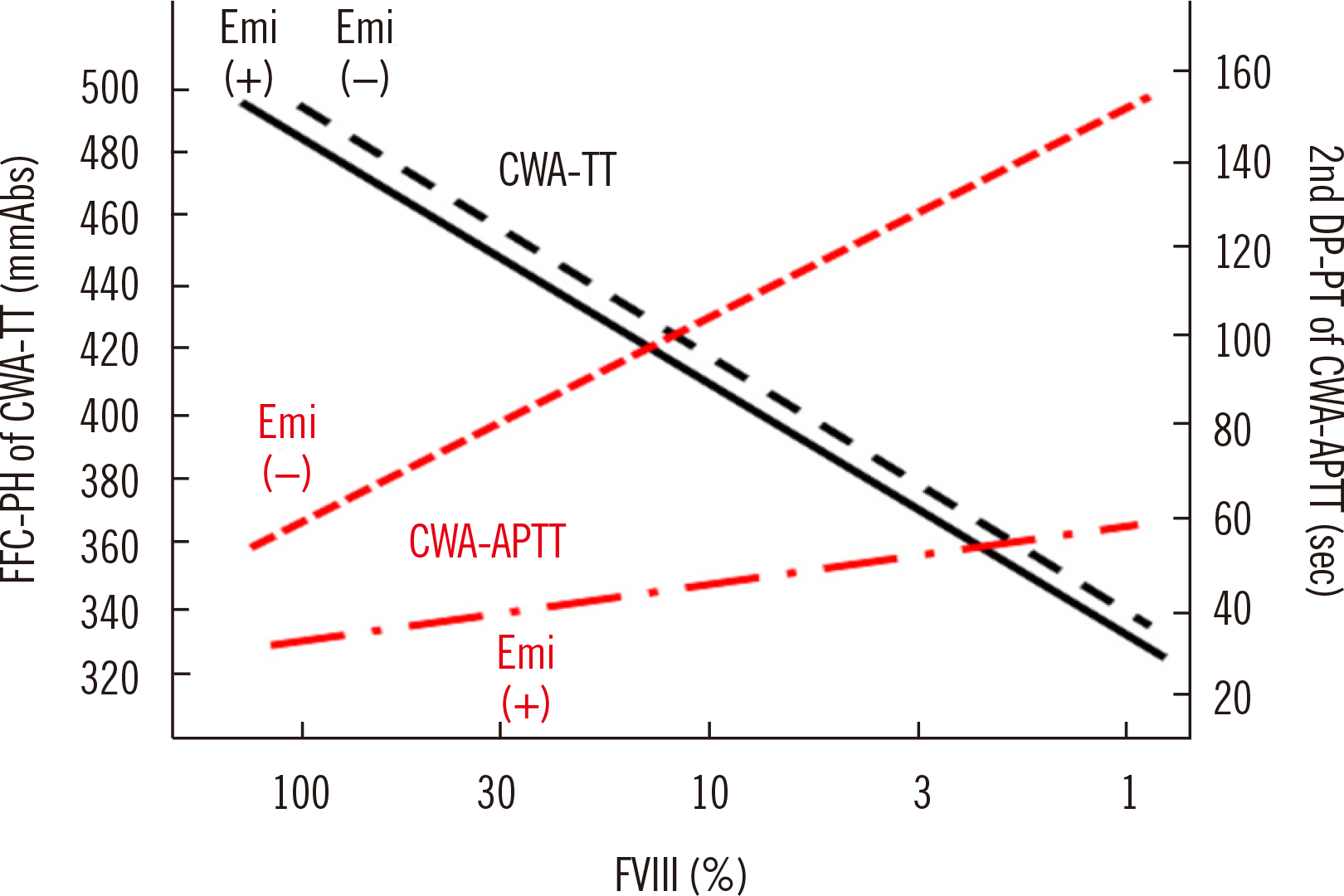
- Clot waveform analysis (CWA) observes changes in transparency in a plasma sample based on clotting tests such as activated partial thromboplastin time (APTT), prothrombin time (PT), and thrombin time (TT). Evidence indicates that not only an abnormal waveform but also peak times and heights in derivative curves of CWA are useful for the evaluation of hemostatic abnormalities. Modified CWA, including the PT with APTT reagent, dilute PT (small amount of tissue factor [TF]-induced clotting factor IX [FIX] activation; sTF/FIXa), and dilute TT, has been proposed to evaluate physiological or pathological hemostasis. We review routine and modified CWA and their clinical applications. In CWA-sTF/FIXa, elevated peak heights indicate hypercoagulability in patients with cancer or thrombosis, whereas prolonged peak times indicate hypocoagulability in several conditions, including clotting factor deficiency and thrombocytopenia. CWA-dilute TT reflects the thrombin burst, whereas clot-fibrinolysis waveform analysis reflects both hemostasis and fibrinolysis. The relevance and usefulness of CWA-APTT and modified CWA should be further investigated in various diseases.
Keyword
Figure
Reference
-
1. Wada H, Matsumoto T, Ohishi K, Shiraki K, Shimaoka M. 2020; Update on the clot waveform analysis. Clin Appl Thromb Hemost. 26:1076029620912027. DOI: 10.1177/1076029620912027. PMID: 32862666. PMCID: PMC7466886.
Article2. Sevenet PO, Depasse F. 2017; Clot waveform analysis: where do we stand in 2017? Int J Lab Hematol. 39:561–8. DOI: 10.1111/ijlh.12724. PMID: 28876509.
Article3. Nogami K. Clot waveform analysis for monitoring hemostasis. Semin Thromb Hemost. 2022; doi: 10.1055/s-0042-1756706 (Online ahead of print). DOI: 10.1055/s-0042-1756706.
Article4. Arai S, Kamijo T, Hayashi F, Shinohara S, Arai N, Sugano M, et al. 2021; Screening method for congenital dysfibrinogenemia using clot waveform analysis with the Clauss method. Int J Lab Hematol. 43:281–9. DOI: 10.1111/ijlh.13358. PMID: 33030793.
Article5. Wada H, Ichikawa Y, Ezaki M, Matsumoto T, Yamashita Y, Shiraki K, et al. 2021; The reevaluation of thrombin time using a clot waveform analysis. J Clin Med. 10:4840. DOI: 10.3390/jcm10214840. PMID: 34768360. PMCID: PMC8585015.
Article6. Wu Y, Lu Y, Zhang J. 2023; Thrombin generation assay: the present and the future. Blood Coagul Fibrinolysis. 34:1–7. DOI: 10.1097/MBC.0000000000001170. PMID: 36598375.
Article7. Thakkar M, Rose A, Bednarz B. 2022; Thromboelastography in microsurgical reconstruction: a systematic review. JPRAS Open. 32:24–33. DOI: 10.1016/j.jpra.2021.12.005. PMID: 35242985. PMCID: PMC8857410.
Article8. Shima M. 2004; Understanding the hemostatic effects of recombinant factor VIIa by clot wave form analysis. Semin Hematol. 41(Suppl 1):125–31. DOI: 10.1053/j.seminhematol.2003.11.021. PMID: 14872433.
Article9. Matsumoto T, Nogami K, Shima M. 2017; A combined approach using global coagulation assays quickly differentiates coagulation disorders with prolonged aPTT and low levels of FVIII activity. Int J Hematol. 105:174–83. DOI: 10.1007/s12185-016-2108-x. PMID: 27730530.
Article10. Toh CH, Giles AR. 2002; Waveform analysis of clotting test optical profiles in the diagnosis and management of disseminated intravascular coagulation (DIC). Clin Lab Haematol. 24:321–7. DOI: 10.1046/j.1365-2257.2002.00457.x. PMID: 12452811.
Article11. Tokutake T, Baba H, Shimada Y, Takeda W, Sato K, Hiroshima Y, et al. 2016; Exogenous magnesium chloride reduces the activated partial thromboplastin times of lupus anticoagulant-positive patients. PLOS ONE. 11:e0157835. DOI: 10.1371/journal.pone.0157835. PMID: 27355205. PMCID: PMC4927146.
Article12. Matsumoto T, Wada H, Nishioka Y, Nishio M, Abe Y, Nishioka J, et al. 2006; Frequency of abnormal biphasic aPTT clot waveforms in patients with underlying disorders associated with disseminated intravascular coagulation. Clin Appl Thromb Hemost. 12:185–92. DOI: 10.1177/107602960601200206. PMID: 16708120.
Article13. Toh CH, Samis J, Downey C, Walker J, Becker L, Brufatto N, et al. 2002; Biphasic transmittance waveform in the APTT coagulation assay is due to the formation of a Ca(++)-dependent complex of C-reactive protein with very-low-density lipoprotein and is a novel marker of impending disseminated intravascular coagulation. Blood. 100:2522–9. DOI: 10.1182/blood.V100.7.2522. PMID: 12239165.
Article14. Shima M, Matsumoto T, Fukuda K, Kubota Y, Tanaka I, Nishiya K, et al. 2002; The utility of activated partial thromboplastin time (aPTT) clot waveform analysis in the investigation of hemophilia A patients with very low levels of factor VIII activity (FVIII:C). Thromb Haemost. 87:436–41. DOI: 10.1055/s-0037-1613023. PMID: 11916076.
Article15. Maeda K, Wada H, Shinkai T, Tanemura A, Matsumoto T, Mizuno S. 2021; Evaluation of hemostatic abnormalities in patients who underwent major hepatobiliary pancreatic surgery using activated partial thromboplastin time-clot waveform analysis. Thromb Res. 201:154–60. DOI: 10.1016/j.thromres.2021.03.030. PMID: 33862519.
Article16. Winter WE, Greene DN, Beal SG, Isom JA, Manning H, Wilkerson G, et al. 2020; Clotting factors: clinical biochemistry and their roles as plasma enzymes. Adv Clin Chem. 94:31–84. DOI: 10.1016/bs.acc.2019.07.008. PMID: 31952574.
Article17. Al-Amer OM. 2022; The role of thrombin in haemostasis. Blood Coagul Fibrinolysis. 33:145–8. DOI: 10.1097/MBC.0000000000001130. PMID: 35239615.18. Monroe DM, Hoffman M, Roberts HR. 2002; Platelets and thrombin generation. Arterioscler Thromb Vasc Biol. 22:1381–9. DOI: 10.1161/01.ATV.0000031340.68494.34. PMID: 12231555.
Article19. Konstantinidi A, Sokou R, Parastatidou S, Lampropoulou K, Katsaras G, Boutsikou T, et al. 2019; Clinical application of thromboelastography/thromboelastometry (TEG/TEM) in the neonatal population: a narrative review. Semin Thromb Hemost. 45:449–57. DOI: 10.1055/s-0039-1692210. PMID: 31195422.
Article20. Matsumoto T, Wada H, Fujimoto N, Toyoda J, Abe Y MR, Ohishi K, et al. 2018; An evaluation of the activated partial thromboplastin time waveform. Clin Appl Thromb Hemost. 24:764–70. DOI: 10.1177/1076029617724230. PMID: 28884611. PMCID: PMC6714873.
Article21. Katayama H, Matsumoto T, Wada H, Fujimoto N, Toyoda J, Abe Y, et al. 2018; An evaluation of hemostatic abnormalities in patients with hemophilia according to the activated partial thromboplastin time waveform. Clin Appl Thromb Hemost. 24:1170–6. DOI: 10.1177/1076029618757344. PMID: 29439640. PMCID: PMC6714760.
Article22. Wada H, Shiraki K, Matsumoto T, Ohishi K, Shimpo H, Shimaoka M. 2020; Effects of platelet and phospholipids on clot formation activated by a small amount of tissue factor. Thromb Res. 193:146–53. DOI: 10.1016/j.thromres.2020.06.018. PMID: 32559572.
Article23. Hasegawa M, Tone S, Wada H, Naito Y, Matsumoto T, Yamashita Y, et al. 2021; The evaluation of hemostatic abnormalities using a CWA-small amount tissue factor induced FIX activation assay in major orthopedic surgery patients. Clin Appl Thromb Hemost. 27:10760296211012094. DOI: 10.1177/10760296211012094. PMID: 34027710. PMCID: PMC8150457.
Article24. Fan BE, Ng J, Chan SSW, Christopher D, Tso ACY, Ling LM, et al. 2021; COVID-19 associated coagulopathy in critically ill patients: a hypercoagulable state demonstrated by parameters of haemostasis and clot waveform analysis. J Thromb Thrombolysis. 51:663–74. DOI: 10.1007/s11239-020-02318-x. PMID: 33098540. PMCID: PMC7584863.
Article25. Tripodi A. 2016; Thrombin generation assay and its application in the clinical laboratory. Clin Chem. 62:699–707. DOI: 10.1373/clinchem.2015.248625. PMID: 26955824.
Article26. Bendetowicz AV, Kai H, Knebel R, Caplain H, Hemker HC, Lindhout T, et al. 1994; The effect of subcutaneous injection of unfractionated and low molecular weight heparin on thrombin generation in platelet rich plasma-a study in human volunteers. Thromb Haemost. 72:705–12. DOI: 10.1055/s-0038-1648946. PMID: 7900078.
Article27. Wakui M, Fujimori Y, Nakamura S, Kondo Y, Kuroda Y, Oka S, et al. 2019; Distinct features of bivalent direct thrombin inhibitors, hirudin and bivalirudin, revealed by clot waveform analysis and enzyme kinetics in coagulation assays. J Clin Pathol. 72:817–24. DOI: 10.1136/jclinpath-2019-205922. PMID: 31366633.
Article28. Wakui M, Fujimori Y, Katagiri H, Nakamura S, Kondo Y, Kuroda Y, et al. 2019; Assessment of in vitro effects of direct thrombin inhibitors and activated factor X inhibitors through clot waveform analysis. J Clin Pathol. 72:244–50. DOI: 10.1136/jclinpath-2018-205517. PMID: 30518630.
Article29. Wada H, Shiraki K, Matsumoto T, Ohishi K, Shimpo H, Sakano Y, et al. 2021; The evaluation of APTT reagents in reference plasma, recombinant FVIII products; Kovaltry® and Jivi® using CWA, including sTF/7FIX assay. Clin Appl Thromb Hemost. 27:1076029620976913. DOI: 10.1177/1076029620976913. PMID: 33606948. PMCID: PMC7900842.30. Bourguignon A, Tasneem S, Hayward CPM. 2022; Update on platelet procoagulant mechanisms in health and in bleeding disorders. Int J Lab Hematol. 44(Suppl 1):89–100. DOI: 10.1111/ijlh.13866. PMID: 36074709.
Article31. Wada H, Ichikawa Y, Ezaki M, Shiraki K, Moritani I, Yamashita Y, et al. 2021; Clot waveform analysis demonstrates low blood coagulation ability in patients with idiopathic thrombocytopenic purpura. J Clin Med. 10:5987. DOI: 10.3390/jcm10245987. PMID: 34945283. PMCID: PMC8705019.
Article32. Nogami K, Matsumoto T, Tabuchi Y, Soeda T, Arai N, Kitazawa T, et al. 2018; Modified clot waveform analysis to measure plasma coagulation potential in the presence of the anti-factor IXa/factor X bispecific antibody emicizumab. J Thromb Haemost. 16:1078–88. DOI: 10.1111/jth.14022. PMID: 29645406.33. Ogiwara K, Furukawa S, Shinohara S, Tabuchi Y, Arai N, Noguchi-Sasaki M, et al. 2023; Anti-idiotype monoclonal antibodies against emicizumab enable accurate procoagulant and anticoagulant assays, irrespective of the test base, in the presence of emicizumab. Haemophilia. 29:329–35. DOI: 10.1111/hae.14662. PMID: 36137299.
Article34. Kumano O, Suzuki S, Yamazaki M, An Y, Yasaka M, Ieko M, et al. 2023; Evaluation of newly-developed modified diluted prothrombin time reagent in non-valvular atrial fibrillation patients with direct oral anticoagulants: a comparative study with conventional reagents. Int J Lab Hematol. 45:119–25. DOI: 10.1111/ijlh.13971. PMID: 36114152.
Article35. Wada H, Matsumoto T, Yamashita Y, Ohishi K, Ikejiri M, Katayama N. 2019; Routine measurements of factor VIII activity and inhibitor titer in the presence of emicizumab utilizing anti-idiotype monoclonal antibodies: comment. J Thromb Haemost. 17:555–6. DOI: 10.1111/jth.14395. PMID: 30672649.
Article36. Onishi T, Shimonishi N, Takeyama M, Furukawa S, Ogiwara K, Nakajima Y, et al. 2022; The balance of comprehensive coagulation and fibrinolytic potential is disrupted in patients with moderate to severe COVID-19. Int J Hematol. 115:826–37. DOI: 10.1007/s12185-022-03308-w. PMID: 35171446. PMCID: PMC8852977.
Article37. Nogami K, Matsumoto T, Sasai K, Ogiwara K, Arai N, Shima M. 2019; A novel simultaneous clot-fibrinolysis waveform analysis for assessing fibrin formation and clot lysis in haemorrhagic disorders. Br J Haematol. 187:518–29. DOI: 10.1111/bjh.16111. PMID: 31335970.
Article38. Kobayashi M, Wada H, Fukui S, Mizutani H, Ichikawa Y, Shiraki K, et al. 2021; A clot waveform analysis showing a hypercoagulable state in patients with malignant neoplasms. J Clin Med. 10:5352. DOI: 10.3390/jcm10225352. PMID: 34830633. PMCID: PMC8618625.
Article39. Menegatti M, Palla R. 2020; Clinical and laboratory diagnosis of rare coagulation disorders (RCDs). Thromb Res. 196:603–8. DOI: 10.1016/j.thromres.2019.09.006. PMID: 31515069.
Article40. Lowe A, Kitchen S, Jennings I, Kitchen DP, Woods TAL, Walker ID. 2020; Effects of emicizumab on APTT, FVIII assays and FVIII inhibitor assays using different reagents: results of a UK NEQAS proficiency testing exercise. Haemophilia. 26:1087–91. DOI: 10.1111/hae.14177. PMID: 33094895.
Article41. Wada H, Shiraki K, Matsumoto T, Suzuki K, Yamashita Y, Tawara I, et al. 2022; A clot waveform analysis of thrombin time using a small amount of thrombin is useful for evaluating the clotting activity of plasma independent of the presence of emicizumab. J Clin Med. 11:6142. DOI: 10.3390/jcm11206142. PMID: 36294464. PMCID: PMC9605059.
Article42. Onishi T, Ishihara T, Nogami K. 2021; Coagulation and fibrinolysis balance in disseminated intravascular coagulation. Pediatr Int. 63:1311–8. DOI: 10.1111/ped.14684. PMID: 33660897.
Article43. Kamon T, Horie S, Inaba T, Ito N, Shiraki K, Ichikawa Y, et al. 2023; The detection of hypercoagulability in patients with acute cerebral infarction using a clot waveform analysis. Clin Appl Thromb Hemost. 29:10760296231161591. DOI: 10.1177/10760296231161591. PMID: 36872898. PMCID: PMC9989368.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Advances in laboratory assessment of thrombosis and hemostasis
- Global hemostatic assay of different target procoagulant activities of factor VIII and factor IX
- Chitosan Pad, Cellulose Membrane, or Gelatin Sponge for Peridural Bleeding: An Efficacy Study on a Lumbar Laminectomized Rat Model
- Spatiotemporal Analysis of Retinal Waveform using Independent Component Analysis in Normal and rd/rd Mouse
- Measurement of FVIII:C Level by Clot Waveform Analysis