J Korean Med Sci.
2024 Feb;39(5):e50. 10.3346/jkms.2024.39.e50.
The Risk of Hypertension and Diabetes Mellitus According to Offspring’s Birthweight in Women With Normal Body Mass Index: A Nationwide Population-Based Study
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Korea University College of Medicine, Seoul, Korea
- 2Big Data Department, National Health Insurance Service, Wonju, Korea
- KMID: 2551979
- DOI: http://doi.org/10.3346/jkms.2024.39.e50
Abstract
- Background
Maladaptation to vascular, metabolic, and physiological changes during pregnancy can lead to fetal growth disorders. Moreover, adverse outcomes during pregnancy can further increase the risk of cardiovascular and metabolic diseases in mothers. Delivering a large-for-gestational-age (LGA) baby may indicate a pre-existing metabolic dysfunction, whereas delivering a small-for-gestational-age (SGA) baby may indicate a pre-existing vascular dysfunction. This study aims to assess the risk of hypertension (HTN) and diabetes mellitus (DM) in women with normal body mass index (BMI) scores who did not experience gestational DM or hypertensive disorders during pregnancy based on the offspring’s birthweight.
Methods
This retrospective nationwide study included women with normal BMI scores who delivered a singleton baby after 37 weeks. Women with a history of DM or HTN before pregnancy and those with gestational DM or hypertensive disorders, were excluded from the study. We compared the risk of future maternal outcomes (HTN and DM) according to the offspring’s birthweight. Multivariate analyses were performed to estimate the hazard ratio (HR) for the future risk of HTN or DM.
Results
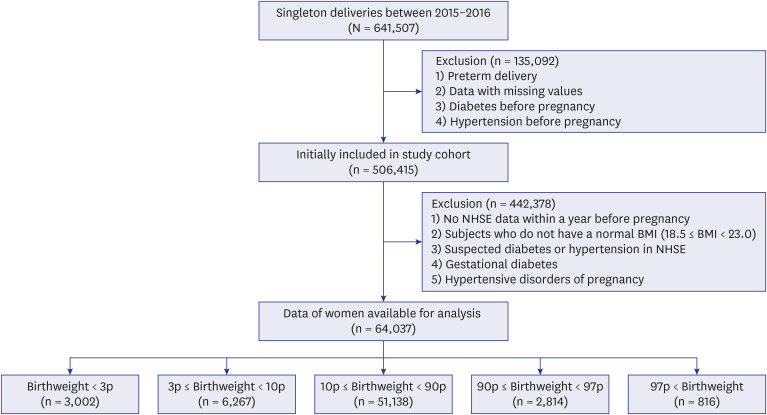
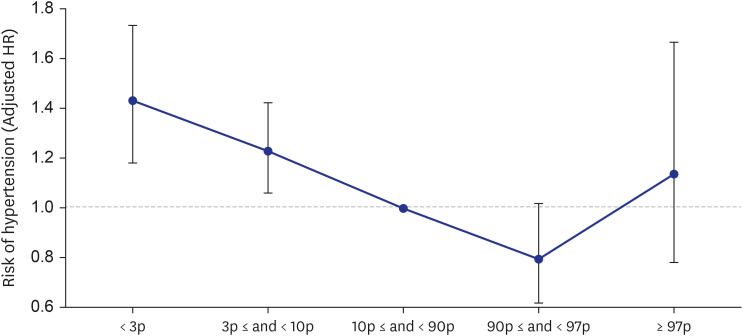
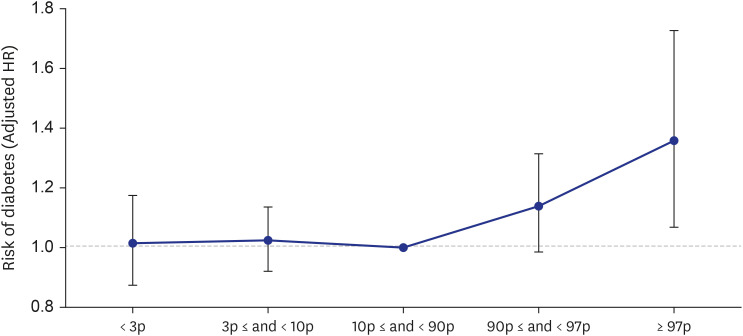
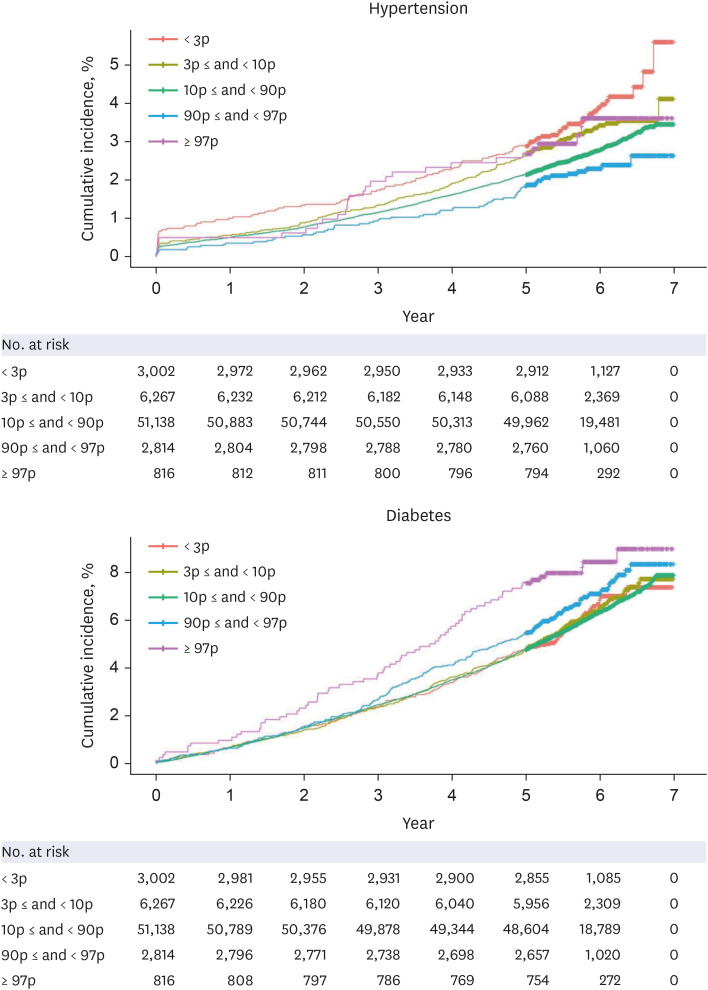
A total of 64,037 women were included in the analysis. Of these, women who delivered very LGA babies (birthweight > 97th percentile) were at a higher risk of developing DM than those who delivered appropriate-for-gestational-age (AGA) babies (adjusted HR = 1.358 [1.068–1.727]), and women who delivered very SGA babies (birthweight < 3rd percentile) were at a higher risk of developing HTN than those who delivered AGA babies (adjusted HR = 1.431 [1.181–1.734]), even after adjusting for age, parity, gestational age at delivery, fetal sex, maternal BMI score, and a history of smoking.
Conclusion
These findings provide a novel support for the use of the offspring’s birthweight as a predictor of future maternal diseases such as HTN and DM.
Figure
Reference
-
1. Parikh NI, Gonzalez JM, Anderson CAM, Judd SE, Rexrode KM, Hlatky MA, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American Heart Association. Circulation. 2021; 143(18):e902–e916. PMID: 33779213.2. Berends AL, de Groot CJ, Sijbrands EJ, Sie MP, Benneheij SH, Pal R, et al. Shared constitutional risks for maternal vascular-related pregnancy complications and future cardiovascular disease. Hypertension. 2008; 51(4):1034–1041. PMID: 18259037.3. Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013; 28(1):1–19. PMID: 23397514.4. Hall PS, Nah G, Vittinghoff E, Parker DR, Manson JE, Howard BV, et al. Relation of pregnancy loss to risk of cardiovascular disease in parous postmenopausal women (from the women’s health initiative). Am J Cardiol. 2019; 123(10):1620–1625. PMID: 30871746.5. Tobias DK, Hu FB, Forman JP, Chavarro J, Zhang C. Increased risk of hypertension after gestational diabetes mellitus: findings from a large prospective cohort study. Diabetes Care. 2011; 34(7):1582–1584. PMID: 21593289.6. Parikh NI, Norberg M, Ingelsson E, Cnattingius S, Vasan RS, Domellöf M, et al. Association of pregnancy complications and characteristics with future risk of elevated blood pressure: the Västerbotten intervention program. Hypertension. 2017; 69(3):475–483. PMID: 28137991.7. Kim C, Cheng YJ, Beckles GL. Cardiovascular disease risk profiles in women with histories of gestational diabetes but without current diabetes. Obstet Gynecol. 2008; 112(4):875–883. PMID: 18827131.8. Fraser A, Nelson SM, Macdonald-Wallis C, Cherry L, Butler E, Sattar N, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the avon longitudinal study of parents and children. Circulation. 2012; 125(11):1367–1380. PMID: 22344039.9. Horn J, Tanz LJ, Stuart JJ, Markovitz AR, Skurnik G, Rimm EB, et al. Early or late pregnancy loss and development of clinical cardiovascular disease risk factors: a prospective cohort study. BJOG. 2019; 126(1):33–42.10. Öztürk HNO, Türker PF. Fetal programming: could intrauterin life affect health status in adulthood? Obstet Gynecol Sci. 2021; 64(6):473–483. PMID: 34670066.11. Luo ZC, Nuyt AM, Delvin E, Audibert F, Girard I, Shatenstein B, et al. Maternal and fetal IGF-I and IGF-II levels, fetal growth, and gestational diabetes. J Clin Endocrinol Metab. 2012; 97(5):1720–1728. PMID: 22419731.12. HAPO Study Cooperative Research Group. The hyperglycemia and adverse pregnancy outcome (HAPO) study. Int J Gynaecol Obstet. 2002; 78(1):69–77. PMID: 12113977.13. Calabuig-Navarro V, Puchowicz M, Glazebrook P, Haghiac M, Minium J, Catalano P, et al. Effect of ω-3 supplementation on placental lipid metabolism in overweight and obese women. Am J Clin Nutr. 2016; 103(4):1064–1072. PMID: 26961929.14. Gauster M, Hiden U, van Poppel M, Frank S, Wadsack C, Hauguel-de Mouzon S, et al. Dysregulation of placental endothelial lipase in obese women with gestational diabetes mellitus. Diabetes. 2011; 60(10):2457–2464. PMID: 21852675.15. Wright E, Audette MC, Ye XY, Keating S, Hoffman B, Lye SJ, et al. Maternal vascular malperfusion and adverse perinatal outcomes in low-risk nulliparous women. Obstet Gynecol. 2017; 130(5):1112–1120. PMID: 29016509.16. Quant HS, Sammel MD, Parry S, Schwartz N. Second-trimester 3-dimensional placental sonography as a predictor of small-for-gestational-age birth weight. J Ultrasound Med. 2016; 35(8):1693–1702. PMID: 27335442.17. Zur RL, Kingdom JC, Parks WT, Hobson SR. The placental basis of fetal growth restriction. Obstet Gynecol Clin North Am. 2020; 47(1):81–98. PMID: 32008673.18. Reister F, Frank HG, Kingdom JC, Heyl W, Kaufmann P, Rath W, et al. Macrophage-induced apoptosis limits endovascular trophoblast invasion in the uterine wall of preeclamptic women. Lab Invest. 2001; 81(8):1143–1152. PMID: 11502865.19. Sato Y. Endovascular trophoblast and spiral artery remodeling. Mol Cell Endocrinol. 2020; 503:110699. PMID: 31899258.20. Cui Y, Wang W, Dong N, Lou J, Srinivasan DK, Cheng W, et al. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature. 2012; 484(7393):246–250. PMID: 22437503.21. Khalil A, Maiz N, Garcia-Mandujano R, Elkhouli M, Nicolaides KH. Longitudinal changes in maternal corin and mid-regional proatrial natriuretic peptide in women at risk of pre-eclampsia. Ultrasound Obstet Gynecol. 2015; 45(2):190–198. PMID: 25296530.22. Grandi SM, Filion KB, Yoon S, Ayele HT, Doyle CM, Hutcheon JA, et al. Cardiovascular disease-related morbidity and mortality in women with a history of pregnancy complications. Circulation. 2019; 139(8):1069–1079. PMID: 30779636.23. Markovitz AR, Stuart JJ, Horn J, Williams PL, Rimm EB, Missmer SA, et al. Does pregnancy complication history improve cardiovascular disease risk prediction? Findings from the HUNT study in Norway. Eur Heart J. 2019; 40(14):1113–1120. PMID: 30596987.24. Rayes B, Ardissino M, Slob EAW, Patel KHK, Girling J, Ng FS. Association of hypertensive disorders of pregnancy with future cardiovascular disease. JAMA Netw Open. 2023; 6(2):e230034. PMID: 36800181.25. Ngo AD, Roberts CL, Chen JS, Figtree G. Delivery of a small-for-gestational-age infant and risk of maternal cardiovascular disease--a population-based record linkage study. Heart Lung Circ. 2015; 24(7):696–704. PMID: 25697383.26. Salihu HM, Garcia BY, Dongarwar D, Maiyegun SO, Yusuf KK, Agili DE. Maternal pre-pregnancy underweight and the risk of small-for-gestational-age in Asian-American ethnic groups. Obstet Gynecol Sci. 2021; 64(6):496–505. PMID: 34666428.27. Hakkarainen H, Huopio H, Cederberg H, Voutilainen R, Heinonen S. Delivery of an LGA infant and the maternal risk of diabetes: a prospective cohort study. Prim Care Diabetes. 2018; 12(4):364–370. PMID: 29735430.28. Kew S, Ye C, Sermer M, Connelly PW, Hanley AJ, Zinman B, et al. Postpartum metabolic function in women delivering a macrosomic infant in the absence of gestational diabetes mellitus. Diabetes Care. 2011; 34(12):2608–2613. PMID: 21972414.29. Mito A, Arata N, Qiu D, Sakamoto N, Murashima A, Ichihara A, et al. Hypertensive disorders of pregnancy: a strong risk factor for subsequent hypertension 5 years after delivery. Hypertens Res. 2018; 41(2):141–146. PMID: 29093561.30. Wilson BJ, Watson MS, Prescott GJ, Sunderland S, Campbell DM, Hannaford P, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003; 326(7394):845. PMID: 12702615.31. Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998; 16(1):5–15. PMID: 9654603.32. Spaanderman ME, Ekhart TH, van Eyck J, Cheriex EC, de Leeuw PW, Peeters LL. Latent hemodynamic abnormalities in symptom-free women with a history of preeclampsia. Am J Obstet Gynecol. 2000; 182(1 Pt 1):101–107. PMID: 10649163.33. Possomato-Vieira JS, Khalil RA. Mechanisms of endothelial dysfunction in hypertensive pregnancy and preeclampsia. Adv Pharmacol. 2016; 77:361–431. PMID: 27451103.34. Bijl RC, Cornette JMJ, Vasak B, Franx A, Lely AT, Bots ML, et al. Cardiometabolic profiles in women with a history of hypertensive and normotensive fetal growth restriction. J Womens Health (Larchmt). 2022; 31(1):63–70. PMID: 34520259.35. Moses R, Davis W, Rodgers D, Meyer B, Calvert D. The metabolic profile of glucose tolerant women who have had large for gestational age babies. Aust N Z J Obstet Gynaecol. 1997; 37(2):177–180. PMID: 9222462.36. Kabeya Y, Goto A, Kato M, Takahashi Y, Matsushita Y, Inoue M, et al. History of having a macrosomic infant and the risk of diabetes: the Japan public health center-based prospective diabetes study. PLoS One. 2013; 8(12):e84542. PMID: 24367673.37. James-Todd TM, Karumanchi SA, Hibert EL, Mason SM, Vadnais MA, Hu FB, et al. Gestational age, infant birth weight, and subsequent risk of type 2 diabetes in mothers: Nurses’ Health Study II. Prev Chronic Dis. 2013; 10:E156. PMID: 24050526.38. Rich-Edwards JW, McElrath TF, Karumanchi SA, Seely EW. Breathing life into the lifecourse approach: pregnancy history and cardiovascular disease in women. Hypertension. 2010; 56(3):331–334. PMID: 20679178.39. Cortés YI, Catov JM, Brooks M, Harlow SD, Isasi CR, Jackson EA, et al. History of adverse pregnancy outcomes, blood pressure, and subclinical vascular measures in late midlife: SWAN (Study of Women’s Health Across the Nation). J Am Heart Assoc. 2017; 7(1):e007138. PMID: 29288157.40. Catov JM, Dodge R, Yamal JM, Roberts JM, Piller LB, Ness RB. Prior preterm or small-for-gestational-age birth related to maternal metabolic syndrome. Obstet Gynecol. 2011; 117(2 Pt 1):225–232. PMID: 21252733.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Risk and Risk Factors for Postpartum Type 2 Diabetes Mellitus in Women with Gestational Diabetes: A Korean Nationwide Cohort Study
- Relation of placental size to large-for-gestational-age infants in women with gestational diabetes mellitus controlled with insulin
- Perception of Risk of Developing Diabetes in Offspring of Type 2 Diabetic Patients
- Gestational Diabetes in Korea: Incidence and Risk Factors of Diabetes in Women with Previous Gestational Diabetes
- Glucose Tolerance and Insulin Secretion patterns by Body Mass Index(BMI) in Offspring of Parents with Non-Insulin Dependent Diabetes Mellitus