J Korean Med Sci.
2024 Feb;39(5):e43. 10.3346/jkms.2024.39.e43.
Realistic Estimation of COVID-19 Infection by Seroprevalence Surveillance of SARS-CoV-2 Antibodies: An Experience From Korea Metropolitan Area From January to May 2022
- Affiliations
-
- 1Department of Laboratory Medicine, Dong-A University Hospital, Dong-A University College of Medicine, Busan, Korea
- 2Department of Social and Preventive Medicine, Sungkyunkwan University School of Medicine, Suwon, Korea
- 3Department of Laboratory Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Department of Laboratory Medicine, International St. Mary’s Hospital, College of Medicine, Catholic Kwandong University, Incheon, Korea
- 5Department of Laboratory Medicine, Dongkuk University Medical Center, Goyang, Korea
- 6Division of Clinical Vaccine Research, Center for Vaccine Research, National Institute of Infectious Diseases, National Institute of Health, Korea Disease Control and Prevention Agency, Cheongju, Korea
- 7Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2551975
- DOI: http://doi.org/10.3346/jkms.2024.39.e43
Abstract
- Background
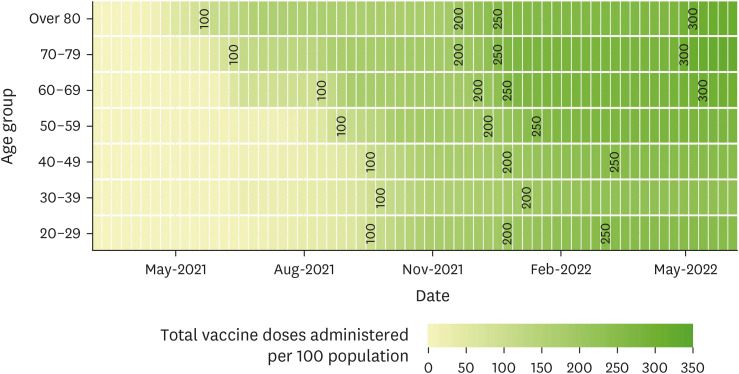
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread globally, leading to the coronavirus disease 2019 (COVID-19) pandemic. Because a significant proportion of the COVID-19 confirmed cases were concentrated in the capital metropolitan area of South Korea, and a large proportion of the population in the area had been adequately vaccinated against COVID-19, we conducted a seroprevalence surveillance study focusing on the residents of the capital metropolitan area in South Korea.
Methods
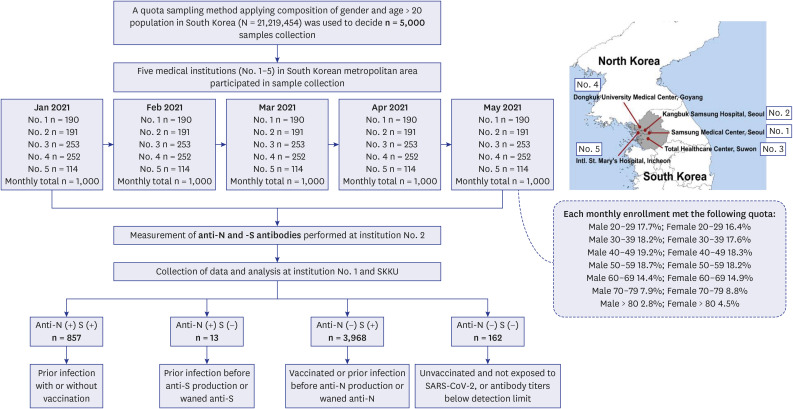
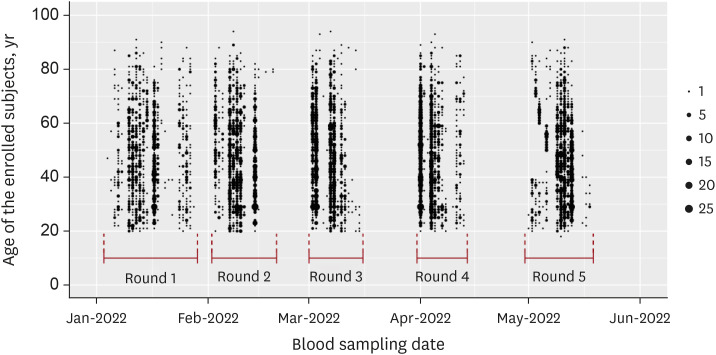
We used a quota-sampling method to obtain blood samples from 1,000 individuals per round, equally stratified across seven age categories and sexes and regions, from five medical institutions located within the capital metropolitan area of South Korea. During five consecutive months (rounds) between January 2022 and May 2022, a total of 5,000 samples were analyzed for anti-spike (S) and anti-nucleocapsid (N) antibodies.
Results
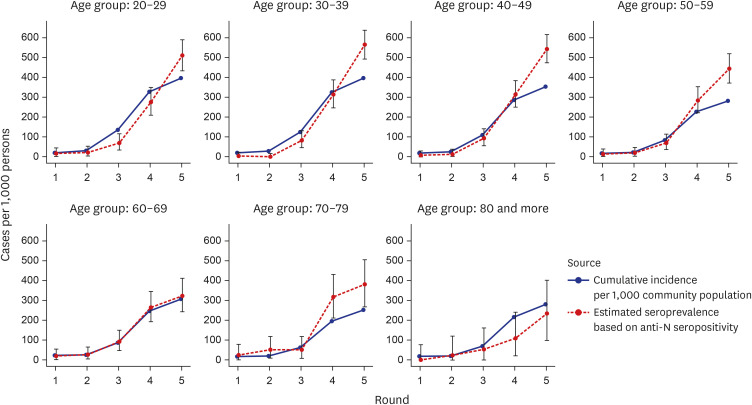
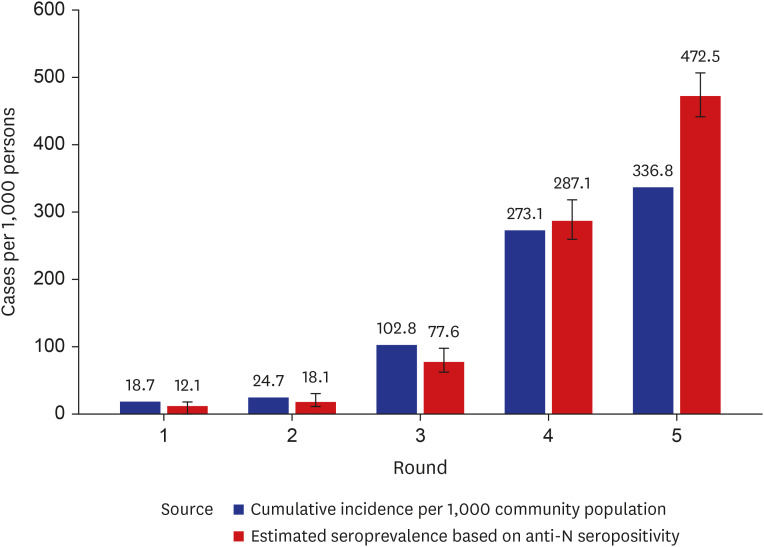
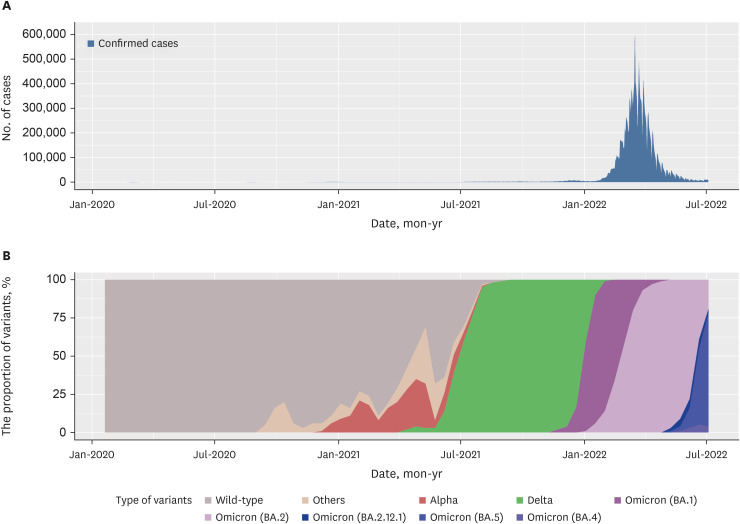
High anti-S seropositivity was observed in all age groups, which corresponded to the vaccine coverage during the study period. Both the cumulative incidence based on polymerase chain reaction (PCR) and the estimated seroprevalence based on anti-N seropositivity increased in the fourth and fifth rounds, which corresponded to April 2022 and May 2022. Seroprevalence coincided with the cumulative incidence during the first three rounds, but exceeded from the fourth survey onwards when infection with omicron variants was increased rapidly in Korea.
Conclusion
Seroprevalence confirmed the number of infection cases outside of PCR testing-based surveillance. Seroepidemiological surveillance can help us understand vaccine responses and detect hidden infections, thereby providing appropriate public health guidance for achieving population-level immunity.
Figure
Reference
-
1. Korea Disease Control and Prevention Agency. Coronavirus disease 19 (population data only available in Korean). Updated 2021. Accessed March 30, 2023. https://ncov.kdca.go.kr/ .2. Kim JY, Choe PG, Oh Y, Oh KJ, Kim J, Park SJ, et al. The first case of 2019 novel coronavirus pneumonia imported into Korea from Wuhan, China: implication for infection prevention and control measures. J Korean Med Sci. 2020; 35(5):e61. PMID: 32030925.3. Hong KH, Lee SW, Kim TS, Huh HJ, Lee J, Kim SY, et al. Guidelines for laboratory diagnosis of coronavirus disease 2019 (COVID-19) in Korea. Ann Lab Med. 2020; 40(5):351–360. PMID: 32237288.4. Johansson MA, Quandelacy TM, Kada S, Prasad PV, Steele M, Brooks JT, et al. SARS-CoV-2 transmission from people without COVID-19 symptoms. JAMA Netw Open. 2021; 4(1):e2035057. PMID: 33410879.5. Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection : a narrative review. Ann Intern Med. 2020; 173(5):362–367. PMID: 32491919.6. World Health Organization. Coronavirus disease (COVID-19): serology, antibodies and immunity. Updated 2020. Accessed March 30, 2023. https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-covid-19-serology .7. Centers for Disease Control and Prevention. Recommendations for use of antibody tests. Updated 2022. Accessed March 30, 2023. https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing/antibody-tests-guidelines.html#AntibodyTests .8. Song SK, Lee DH, Nam JH, Kim KT, Do JS, Kang DW, et al. IgG seroprevalence of COVID-19 among Individuals without a history of the coronavirus disease infection in Daegu, Korea. J Korean Med Sci. 2020; 35(29):e269. PMID: 32715672.9. Noh JY, Seo YB, Yoon JG, Seong H, Hyun H, Lee J, et al. Seroprevalence of anti-SARS-CoV-2 antibodies among outpatients in southwestern Seoul, Korea. J Korean Med Sci. 2020; 35(33):e311. PMID: 32830472.10. Kim AR, Minn D, Kim SH, Do HN, Kim B, Choi YS, et al. Seroprevalence of SARS-CoV-2 antibodies in the community based on participants in the 2020 Korea National Health and Nutrition Examination Survey. Epidemiol Health. 2022; 44:e2022028. PMID: 35209706.11. Yoon K, Kim J, Peck KR, Kim HS, Lee H, Hwang YS, et al. Seroprevalence of SARS-CoV-2 antibodies during the third wave of COVID-19 in the Seoul metropolitan area of Korea. Epidemiol Health. 2022; 44:e2022085. PMID: 36228670.12. Nah EH, Cho S, Park H, Hwang I, Cho HI. Nationwide seroprevalence of antibodies to SARS-CoV-2 in asymptomatic population in South Korea: a cross-sectional study. BMJ Open. 2021; 11(4):e049837.13. World Health Organization. WHO COVID-19 dashboard. Updated 2023. Accessed March 30, 2023. https://covid19.who.int .14. Jang EJ, Choe YJ, Choe SA, Kim YY, Kim RK, Kim J, et al. Presumed population immunity to SARS-CoV-2 in South Korea, April 2022. Osong Public Health Res Perspect. 2022; 13(5):377–381. PMID: 36328242.15. Ministry of the Interior and Safety of South Korea. Resident registration population and household status. Updated 2022. Accessed March 30, 2023. https://jumin.mois.go.kr .16. Duarte N, Yanes-Lane M, Arora RK, Bobrovitz N, Liu M, Bego MG, et al. Adapting serosurveys for the SARS-CoV-2 vaccine era. Open Forum Infect Dis. 2021; 9(2):ofab632. PMID: 35103246.17. Roche Diagnostics. Elecsys® Anti-SARS-CoV-2 S. Updated 2023. Accessed March 30, 2023. https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2-s.html .18. Hong KH, Kim GJ, Roh KH, Sung H, Lee J, Kim SY, et al. Update of guidelines for laboratory diagnosis of COVID-19 in Korea. Ann Lab Med. 2022; 42(4):391–397. PMID: 35177559.19. Sung H, Roh KH, Hong KH, Seong MW, Ryoo N, Kim HS, et al. COVID-19 molecular testing in Korea: practical essentials and answers from experts based on experiences of emergency use authorization assays. Ann Lab Med. 2020; 40(6):439–447. PMID: 32539299.20. Henrion MY. bootComb—an R package to derive confidence intervals for combinations of independent parameter estimates. Int J Epidemiol. 2021; 50(4):1071–1076.21. Reiczigel J, Földi J, Ozsvári L. Exact confidence limits for prevalence of a disease with an imperfect diagnostic test. Epidemiol Infect. 2010; 138(11):1674–1678. PMID: 20196903.22. Jung J. Preparing for the coronavirus disease (COVID-19) vaccination: evidence, plans, and implications. J Korean Med Sci. 2021; 36(7):e59. PMID: 33619920.23. Castilla J, Lecea Ó, Martín Salas C, Quílez D, Miqueleiz A, Trobajo-Sanmartín C, et al. Seroprevalence of antibodies against SARS-CoV-2 and risk of COVID-19 in Navarre, Spain, May to July 2022. Euro Surveill. 2022; 27(33):2200619. PMID: 35983774.24. Reedman CN, Drews SJ, Yi QL, Pambrun C, O’Brien SF. Changing patterns of SARS-CoV-2 seroprevalence among Canadian blood donors during the vaccine era. Microbiol Spectr. 2022; 10(2):e0033922. PMID: 35412385.25. Clarke KE, Jones JM, Deng Y, Nycz E, Lee A, Iachan R, et al. Seroprevalence of infection-induced SARS-CoV-2 antibodies - United States, September 2021-February 2022. MMWR Morb Mortal Wkly Rep. 2022; 71(17):606–608. PMID: 35482574.26. Han J, Baek HJ, Noh E, Yoon K, Kim JA, Ryu S, et al. Korea Seroprevalence Study of Monitoring of SARS-CoV-2 Antibody Retention and Transmission (K-SEROSMART): findings from national representative sample. Epidemiol Health. 2023; 45:e2023075. PMID: 37591786.27. Andreano E, Paciello I, Piccini G, Manganaro N, Pileri P, Hyseni I, et al. Hybrid immunity improves B cells and antibodies against SARS-CoV-2 variants. Nature. 2021; 600(7889):530–535. PMID: 34670266.28. Altarawneh HN, Chemaitelly H, Ayoub HH, Tang P, Hasan MR, Yassine HM, et al. Effects of previous infection and vaccination on symptomatic omicron infections. N Engl J Med. 2022; 387(1):21–34. PMID: 35704396.29. Hall V, Foulkes S, Insalata F, Kirwan P, Saei A, Atti A, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. 2022; 386(13):1207–1220. PMID: 35172051.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- COVID-19 and Breastfeeding
- The Seroprevalence of SARS-CoV-2 in Children During Early COVID-19 Pandemic in Korea: A Nationwide, Population-Based Study
- Seroprevalence of immunoglobulin G antibodies against SARS-CoV-2 in children and adolescents in Delhi, India, from January to October 2021: a repeated cross-sectional analysis
- Evaluation of Seroprevalence of SARS-CoV-2 IgG in Healthcare Workers in a Tertiary Hospital in Seoul
- The Broad Spectrum of Neuro-Radiological Abnormalities in Patients Infected with SARS-CoV-2 Supports the Diagnosis of Neuro-COVID-19