J Korean Med Sci.
2024 Jan;39(4):e38. 10.3346/jkms.2024.39.e38.
Prevalence and Burden of Human Adenovirus-Associated Acute Respiratory Illness in the Republic of Korea Military, 2013 to 2022
- Affiliations
-
- 1Department of Critical Care Medicine, Department of Internal Medicine, Armed Forces Capital Hospital, Seongnam, Korea
- 2Department of Laboratory Medicine, Armed Forces Capital Hospital, Seongnam, Korea
- 3Department of Preventive Medicine, College of Medicine, Chung-Ang University, Seoul, Korea
- 4Department of Infectious Diseases Research, Armed Forces Medical Research Institute, Daejeon, Korea
- 5Division of Infectious Disease, Department of Internal Medicine, Hallym University Sacred Heart Hospital, Anyang, Korea
- 6Division of Infectious Diseases, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 7Department of Preventive Medicine, College of Medicine, Graduate Program for System Health Science and Engineering, Ewha Womans University, Seoul, Korea
- 8Department of Preventive Medicine, Gachon University College of Medicine, Incheon, Korea
- 9Artificial Intelligence and Big-Data Convergence Centre, Gil Medical Centre, Gachon University College of Medicine, Incheon, Korea
- KMID: 2551968
- DOI: http://doi.org/10.3346/jkms.2024.39.e38
Abstract
- Background
Human adenovirus (HAdV) is a common cause of acute respiratory disease (ARD) and has raised significant concerns within the Korean military. Here, we conducted a comprehensive epidemiological analysis of HAdV-associated ARD by evaluating its prevalence, clinical outcomes, and prognosis.
Methods
We reviewed data from multiple sources, including the New Defense Medical Information System, Defense Medical Statistical Information System, Ministry of National Defense, Army Headquarters, Navy Headquarters, Air Force Headquarters, and Armed Forces Medical Command. We analyzed data of patients who underwent polymerase chain reaction (PCR) testing for respiratory viruses between January 2013 and July 2022 in all 14 Korean military hospitals. The analysis included the PCR test results, demographic characteristics, health care utilization, and prognosis including types of treatments received, incidence of pneumonia, and mortality.
Results
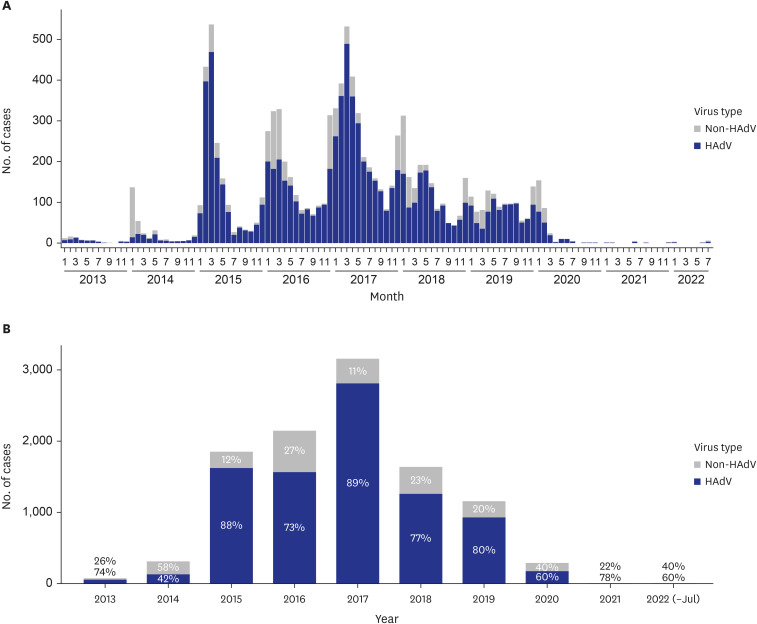
Among the 23,830 individuals who underwent PCR testing at Korean military hospitals, 44.78% (10,670 cases) tested positive for respiratory viruses. Across all military types and ranks, HAdV was the most prevalent virus, with a total of 8,580 patients diagnosed, among HAdV, influenza virus, human metapneumovirus, human parainfluenza virus, and human respiratory syncytial virus. HAdV-infected patients exhibited higher rates of healthcare use compared to non-HAdV-infected patients, including a greater number of emergency visits (1.04 vs. 1.02) and outpatient visits (1.31 vs. 1.27), longer hospitalizations (8.14 days vs. 6.84 days), and extended stays in the intensive care unit (5.21 days vs. 3.38 days). Furthermore, HAdV-infected patients had a higher proportion of pneumonia cases (65.79%vs. 48.33%) and greater likelihood of receiving advanced treatments such as high flow nasal cannula or continuous renal replacement therapy.
Conclusion
Our findings indicate that HAdV posed a significant public health concern within the Korean military prior to the coronavirus disease 2019 (COVID-19) pandemic. Given the potential for a resurgence of outbreaks in the post-COVID-19 era, proactive measures, such as education, environmental improvements, and the development of HAdV vaccines, are crucial for effectively preventing future outbreaks.
Figure
Reference
-
1. Gaydos CA, Gaydos JC. Adenovirus vaccines in the U.S. military. Mil Med. 1995; 160(6):300–304. PMID: 7659229.2. Radin JM, Hawksworth AW, Blair PJ, Faix DJ, Raman R, Russell KL, et al. Dramatic decline of respiratory illness among US military recruits after the renewed use of adenovirus vaccines. Clin Infect Dis. 2014; 59(7):962–968. PMID: 24991024.3. Yoon H, Jhun BW, Kim H, Yoo H, Park SB. Characteristics of adenovirus pneumonia in Korean military personnel, 2012–2016. J Korean Med Sci. 2017; 32(2):287–295. PMID: 28049240.4. Heo JY, Noh JY, Jeong HW, Choe KW, Song JY, Kim WJ, et al. Molecular epidemiology of human adenovirus-associated febrile respiratory illness in soldiers, South Korea. Emerg Infect Dis. 2018; 24(7):1221–1227. PMID: 29912713.5. Park JY, Kim BJ, Lee EJ, Park KS, Park HS, Jung SS, et al. Clinical features and courses of adenovirus pneumonia in healthy young adults during an outbreak among Korean military personnel. PLoS One. 2017; 12(1):e0170592. PMID: 28114362.6. Ko JH, Woo HT, Oh HS, Moon SM, Choi JY, Lim JU, et al. Ongoing outbreak of human adenovirus-associated acute respiratory illness in the Republic of Korea military, 2013 to 2018. Korean J Intern Med. 2021; 36(1):205–213. PMID: 31480827.7. Yoo H, Gu SH, Jung J, Song DH, Yoon C, Hong DJ, et al. Febrile respiratory illness associated with human adenovirus type 55 in South Korea military, 2014–2016. Emerg Infect Dis. 2017; 23(6):1016–1020. PMID: 28518038.8. Yoo H, Oh J, Park C. Characteristics of fever and response to antipyretic therapy in military personnel with adenovirus-positive community-acquired pneumonia. Mil Med Res. 2020; 7(1):6. PMID: 32079545.9. Ko JH, Lim JU, Choi JY, Oh HS, Yoo H, Jhun BW, et al. Early cidofovir administration might be associated with a lower probability of respiratory failure in treating human adenovirus pneumonia: a retrospective cohort study. Clin Microbiol Infect. 2020; 26(5):646.e9–646.14.10. Fourgeaud J, Toubiana J, Chappuy H, Delacourt C, Moulin F, Parize P, et al. Impact of public health measures on the post-COVID-19 respiratory syncytial virus epidemics in France. Eur J Clin Microbiol Infect Dis. 2021; 40(11):2389–2395. PMID: 34347190.11. Treggiari D, Piubelli C, Formenti F, Silva R, Perandin F. Resurgence of respiratory virus after relaxation of COVID-19 containment measures: a real-world data study from a regional hospital of Italy. Int J Microbiol. 2022; 2022:4915678. PMID: 36466968.12. Chen B, Zhu Z, Li Q, He D. Resurgence of different influenza types in China and the US in 2021. Math Biosci Eng. 2023; 20(4):6327–6333. PMID: 37161109.13. Hoke CH Jr, Snyder CE Jr. History of the restoration of adenovirus type 4 and type 7 vaccine, live oral (adenovirus vaccine) in the context of the Department of Defense acquisition system. Vaccine. 2013; 31(12):1623–1632. PMID: 23291475.14. Liu MC, Xu Q, Li TT, Wang T, Jiang BG, Lv CL, et al. Prevalence of human infection with respiratory adenovirus in China: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2023; 17(2):e0011151. PMID: 36812245.15. Dongliu Y, Guoliang Y, Haocheng X, Shuaijia Q, Li B, Yanglei J. Outbreak of acute febrile respiratory illness caused by human adenovirus B P14H11F14 in a military training camp in Shandong China. Arch Virol. 2016; 161(9):2481–2489. PMID: 27352268.16. Lu G, Peng X, Li R, Liu Y, Wu Z, Wang X, et al. An outbreak of acute respiratory infection at a training base in Beijing, China due to human adenovirus type B55. BMC Infect Dis. 2020; 20(1):537. PMID: 32703176.17. Kajon AE, Dickson LM, Metzgar D, Houng HS, Lee V, Tan BH. Outbreak of febrile respiratory illness associated with adenovirus 11a infection in a Singapore military training cAMP. J Clin Microbiol. 2010; 48(4):1438–1441. PMID: 20129957.18. Chmielewicz B, Benzler J, Pauli G, Krause G, Bergmann F, Schweiger B. Respiratory disease caused by a species B2 adenovirus in a military camp in Turkey. J Med Virol. 2005; 77(2):232–237. PMID: 16121380.19. Li X, Kong M, Su X, Zou M, Guo L, Dong X, et al. An outbreak of acute respiratory disease in China caused by human adenovirus type B55 in a physical training facility. Int J Infect Dis. 2014; 28:117–122. PMID: 25236387.20. Yi L, Zou L, Lu J, Kang M, Song Y, Su J, et al. A cluster of adenovirus type B55 infection in a neurosurgical inpatient department of a general hospital in Guangdong, China. Influenza Other Respi Viruses. 2017; 11(4):328–336.21. Liu H, Li Q, Xiang Y, Li H, Liu K, Du X, et al. An outbreak of acute respiratory disease caused by HAdV-55 in Beijing, China, 2020. J Med Virol. 2022; 94(12):6111–6115. PMID: 35981961.22. Huh K, Kim I, Jung J, Lee JE, Jhun BW, Gu SH, et al. Prolonged shedding of type 55 human adenovirus in immunocompetent adults with adenoviral respiratory infections. Eur J Clin Microbiol Infect Dis. 2019; 38(4):793–800. PMID: 30693422.23. Hang J, Kajon AE, Graf PC, Berry IM, Yang Y, Sanborn MA, et al. Human adenovirus type 55 distribution, regional persistence, and genetic variability. Emerg Infect Dis. 2020; 26(7):1497–1505. PMID: 32568062.24. Reis J, Shaman J. Simulation of four respiratory viruses and inference of epidemiological parameters. Infect Dis Model. 2018; 3:23–34. PMID: 30839912.25. Tian X, Fan Y, Liu Z, Zhang L, Liao J, Zhou Z, et al. Broadly neutralizing monoclonal antibodies against human adenovirus types 55, 14p, 7, and 11 generated with recombinant type 11 fiber knob. Emerg Microbes Infect. 2018; 7(1):206. PMID: 30531794.26. Tian X, Ma Q, Jiang Z, Huang J, Liu Q, Lu X, et al. Identification and application of neutralizing epitopes of human adenovirus type 55 hexon protein. Viruses. 2015; 7(10):5632–5642. PMID: 26516903.27. Park SY, Ko JH, Monoldorova S, Jeong J, Jeon BY, Kwon SH. Seroprevalence of neutralizing antibodies against human adenovirus type 55 in the South Korean military, 2018-2019. PLoS One. 2020; 15(7):e0236040. PMID: 32673367.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Clinical features of Severe Adenovirus Pneumonia among Members of the Korea Military: A Case Series
- Ongoing outbreak of human adenovirus-associated acute respiratory illness in the Republic of Korea military, 2013 to 2018
- The Changes in Respiratory and Enteric Adenovirus Epidemiology in Korea From 2017 to June 2022
- Severe Adenovirus Pneumonia
- Clinical features of respiratory adenovirus infections in pediatric inpatients in a single medical center