J Korean Med Sci.
2024 Jan;39(1):e8. 10.3346/jkms.2024.39.e8.
Real-World Eligibility and Cost-Effectiveness Analysis of Empagliflozin for Heart Failure in Korea
- Affiliations
-
- 1Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Korea
- 2College of Pharmacy, The Catholic University of Korea, Bucheon, Korea
- 3Division of Cardiology, Department of Internal Medicine, Seoul St. Mary’s Hospital, Catholic Research Institute for Intractable Cardiovascular Disease, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 4Biostatistics Collaboration Unit, Yonsei University College of Medicine, Seoul, Korea
- 5Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 6Seoul National University School of Medicine, Seoul, Korea
- 7Department of Internal Medicine, Sungkyunkwan University College of Medicine, Seoul, Korea
- 8Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 9Department of Internal Medicine, Chungbuk National University Hospital, Cheongju, Korea
- 10Department of Internal Medicine, Kyungpook National University College of Medicine, Daegu, Korea
- 11Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- 12Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 13Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea
- 14Department of Cardiovascular Medicine, Chonnam National University Medical School, Gwangju, Korea
- 15Department of Cardiovascular medicine, Incheon Sejong Hospital, Incheon, Korea
- 16Department of Cardiology, UC San Diego Health System, La Jolla, CA, USA
- KMID: 2550795
- DOI: http://doi.org/10.3346/jkms.2024.39.e8
Abstract
- Background
The US Food and Drug Administration (FDA) and European Medicines Agency (EMA) approved empagliflozin for reducing cardiovascular mortality and heart failure (HF) hospitalization in patients with both HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF). However, limited data are available on the generalizability of empagliflozin to clinical practice. Therefore, we evaluated real-world eligibility and potential cost-effectiveness based on a nationwide prospective HF registry.
Methods
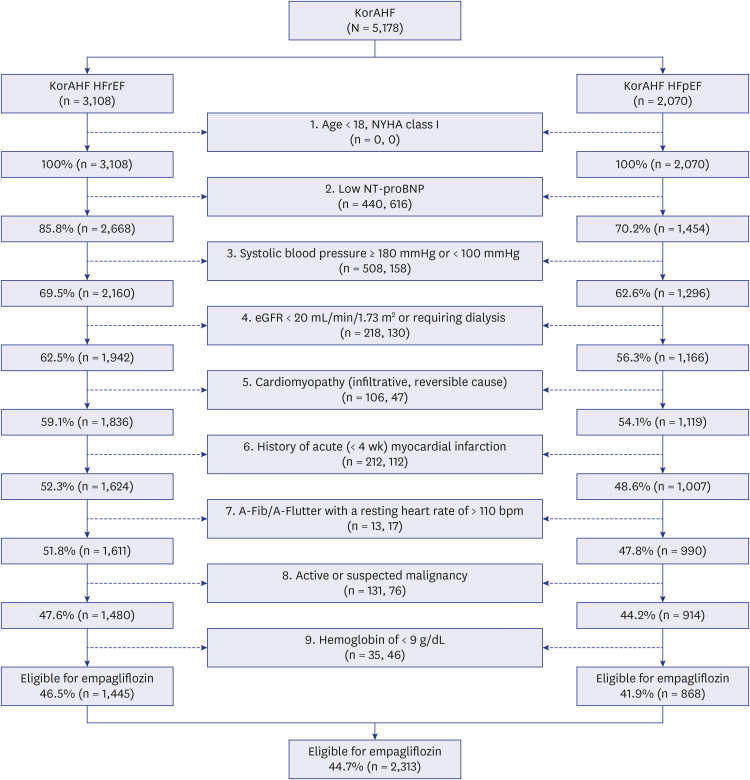
A total of 3,108 HFrEF and 2,070 HFpEF patients from the Korean Acute Heart Failure (KorAHF) registry were analyzed. Eligibility was estimated by inclusion and exclusion criteria of EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Reduced Ejection Fraction (EMPEROR-Reduced) and EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Preserved Ejection Fraction (EMPEROR-Preserved) trials and by FDA & EMA label criteria. The cost-utility analysis was done using a Markov model to project the lifetime medical cost and quality-adjusted life year (QALY).
Results
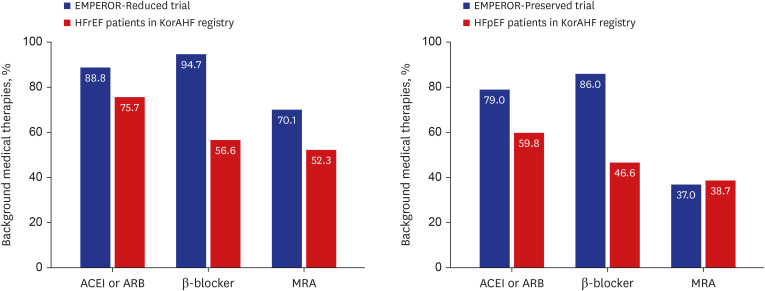
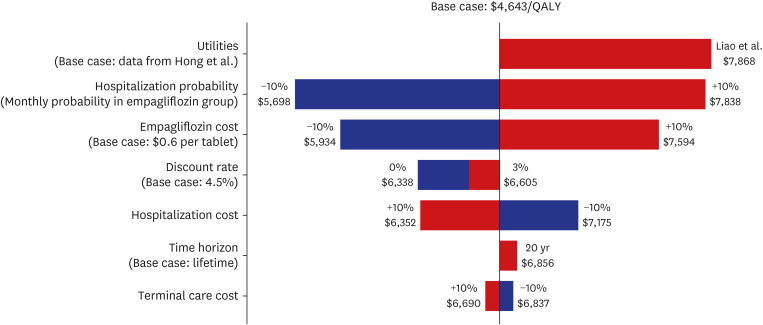
Among the KorAHF patients, 91.4% met FDA & EMA label criteria, while 44.7% met the clinical trial criteria. The incremental cost-effectiveness ratio of empagliflozin was calculated at US$6,764 per QALY in the overall population, which is far below a threshold of US$18,182 per QALY. The cost-effectiveness benefit was more evident in patients with HFrEF (US$5,012 per QALY) than HFpEF (US$8,971 per QALY).
Conclusion
There is a large discrepancy in real-world eligibility for empagliflozin between FDA & EMA labels and clinical trial criteria. Empagliflozin is cost-effective in HF patients regardless of ejection fraction in South Korea health care setting. The efficacy and safety of empagliflozin in real-world HF patients should be further investigated for a broader range of clinical applications.
Figure
Reference
-
1. Kim ES, Youn JC, Baek SH. Update on the pharmacotherapy of heart failure with reduced ejection fraction. Cardiovasc Prev Pharmacother. 2020; 2(4):113–133.2. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. Corrigendum to: 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2021; 42(48):4901. PMID: 34649282.3. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2022; 145(18):e895–1032. PMID: 35363499.4. Hyun J, Cho JY, Youn JC, Kim D, Cho DH, Park SM, et al. Korean Society of Heart Failure guidelines for the management of heart failure: advanced and acute heart failure. Korean Circ J. 2023; 53(7):452–471. PMID: 37525390.5. Youn JC, Han S, Ryu KH. Temporal trends of hospitalized patients with heart failure in Korea. Korean Circ J. 2017; 47(1):16–24. PMID: 28154584.6. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021; 385(16):1451–1461. PMID: 34449189.7. Solomon SD, McMurray JJ, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022; 387(12):1089–1098. PMID: 36027570.8. US Food and Drug Administration. FDA approves treatment for wider range of patients with heart failure. Updated 2022. Accessed August 15, 2022. https://www.fda.gov/news-events/press-announcements/fda-approves-treatment-wider-range-patients-heart-failure .9. European Commission. Union Register of medicinal products for human use. Updated 2022. Accessed 15 August, 2022. https://ec.europa.eu/health/documents/community-register/html/h930.htm .10. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020; 383(15):1413–1424. PMID: 32865377.11. Masoudi FA, Havranek EP, Wolfe P, Gross CP, Rathore SS, Steiner JF, et al. Most hospitalized older persons do not meet the enrollment criteria for clinical trials in heart failure. Am Heart J. 2003; 146(2):250–257. PMID: 12891192.12. Kim JJ, Youn JC. Eligibility and usage of sacubitril/valsartan in Korea. Int J Heart Fail. 2019; 1(1):69–71. PMID: 36262742.13. Lee SE, Cho HJ, Lee HY, Yang HM, Choi JO, Jeon ES, et al. A multicentre cohort study of acute heart failure syndromes in Korea: rationale, design, and interim observations of the Korean Acute Heart Failure (KorAHF) registry. Eur J Heart Fail. 2014; 16(6):700–708. PMID: 24797348.14. Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021; 385(19):1737–1749. PMID: 34554658.15. Song HJ, Lee EK. Evaluation of willingness to pay per quality-adjusted life year for a cure: a contingent valuation method using a scenario-based survey. Medicine (Baltimore). 2018; 97(38):e12453. PMID: 30235732.16. Ahn J, Kim Y, Shin S, Park SY, Song HJ, Park J, et al. Research on Methodologies for Evidence-Based Healthcare Decision-Making Processes in Korea. Seoul, Korea: National Evidence-based Healthcare Collaborating Agency (NECA);2010.17. Seferovic PM, Polovina M, Milinkovic I, Anker S, Rosano G, Coats A. Expect the unexpected in the medical treatment of heart failure with reduced ejection fraction: between scientific evidence and clinical wisdom. Int J Heart Fail. 2021; 3(4):205–218. PMID: 36262556.18. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015; 373(22):2117–2128. PMID: 26378978.19. Oh J, Lee CJ, Park JJ, Lee SE, Kim MS, Cho HJ, et al. Real-world eligibility for sacubitril/valsartan in heart failure with reduced ejection fraction patients in Korea: data from the Korean Acute Heart Failure (KorAHF) registry. Int J Heart Fail. 2019; 1(1):57–68. PMID: 36262737.20. Norberg H, Bergdahl E, Lindmark K. Eligibility of sacubitril-valsartan in a real-world heart failure population: a community-based single-centre study. ESC Heart Fail. 2018; 5(2):337–343. PMID: 29345425.21. Maltês S, Cunha GJ, Rocha BM, Presume J, Guerreiro R, Henriques C, et al. Dapagliflozin in a real-world chronic heart failure population: how many are actually eligible? Cardiology. 2021; 146(2):201–206. PMID: 33524984.22. Arnold SV, Inzucchi SE, Tang F, McGuire DK, Mehta SN, Maddox TM, et al. Real-world use and modeled impact of glucose-lowering therapies evaluated in recent cardiovascular outcomes trials: an NCDR® Research to Practice project. Eur J Prev Cardiol. 2017; 24(15):1637–1645. PMID: 28870145.23. Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996; 275(20):1557–1562. PMID: 8622246.24. Staessen JA, Wang JG, Thijs L. Cardiovascular protection and blood pressure reduction: a meta-analysis. Lancet. 2001; 358(9290):1305–1315. PMID: 11684211.25. Lee TT, Chen J, Cohen DJ, Tsao L. The association between blood pressure and mortality in patients with heart failure. Am Heart J. 2006; 151(1):76–83. PMID: 16368295.26. Kario K, Ferdinand KC, Vongpatanasin W. Are SGLT2 inhibitors new hypertension drugs? Circulation. 2021; 143(18):1750–1753. PMID: 33939525.27. Liao CT, Yang CT, Kuo FH, Lee MC, Chang WT, Tang HJ, et al. Cost-effectiveness evaluation of add-on empagliflozin in patients with heart failure and a reduced ejection fraction from the healthcare system’s perspective in the Asia-Pacific region. Front Cardiovasc Med. 2021; 8:750381. PMID: 34778407.28. Jiang Y, Zheng R, Sang H. Cost-effectiveness of adding SGLT2 inhibitors to standard treatment for heart failure with reduced ejection fraction patients in China. Front Pharmacol. 2021; 12:733681. PMID: 34858172.29. Lin X, Lin M, Liu M, Huang W, Nie X, Chen Z, et al. Cost-effectiveness of empagliflozin as a treatment for heart failure with reduced ejection fraction: an analysis from the Chinese healthcare perspective. J Thorac Dis. 2022; 14(5):1588–1597. PMID: 35693603.30. Krittayaphong R, Permsuwan U. Cost-utility analysis of combination empagliflozin and standard treatment versus standard treatment alone in Thai heart failure patients with reduced or preserved ejection fraction. Am J Cardiovasc Drugs. 2022; 22(5):577–590. PMID: 35796952.31. Teng TK, Tromp J, Tay WT, Anand I, Ouwerkerk W, Chopra V, et al. Prescribing patterns of evidence-based heart failure pharmacotherapy and outcomes in the ASIAN-HF registry: a cohort study. Lancet Glob Health. 2018; 6(9):e1008–18. PMID: 30103979.32. Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF registry. J Am Coll Cardiol. 2018; 72(4):351–366. PMID: 30025570.33. Youn JC, Ahn Y, Jung HO. Pathophysiology of heart failure with preserved ejection fraction. Heart Fail Clin. 2021; 17(3):327–335. PMID: 34051965.34. Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004; 351(6):543–551. PMID: 15295047.35. Böhm M, Anker SD, Butler J, Filippatos G, Ferreira JP, Pocock SJ, et al. Empagliflozin improves cardiovascular and renal outcomes in heart failure irrespective of systolic blood pressure. J Am Coll Cardiol. 2021; 78(13):1337–1348. PMID: 34556320.36. Zannad F, Ferreira JP, Pocock SJ, Zeller C, Anker SD, Butler J, et al. Cardiac and kidney benefits of empagliflozin in heart failure across the spectrum of kidney function: insights from EMPEROR-Reduced. Circulation. 2021; 143(4):310–321. PMID: 33095032.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Empagliflozin on Diuretics Reduction in Outpatient Heart Failure Patients
- Economic evaluation of v accinations - a methodologic review
- Sodium-glucose Co-transporter 2 Inhibitors: a New Path for Heart Failure Treatment
- Real-World Eligibility for Sacubitril/ Valsartan in Heart Failure with Reduced Ejection Fraction Patients in Korea: Data from the Korean Acute Heart Failure (KorAHF) Registry
- A Study on the Method to Estimate the Cost of Hospice Care