J Pathol Transl Med.
2024 Jan;58(1):12-21. 10.4132/jptm.2023.11.02.
Tumor-infiltrating T lymphocytes evaluated using digital image analysis predict the prognosis of patients with diffuse large B-cell lymphoma
- Affiliations
-
- 1Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Pathology, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- 3Department of Pathology, Seoul National University, Seoul National College of Medicine, Seoul, Korea
- 4Division of Hematology and Oncology, Department of Internal Medicine, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2550496
- DOI: http://doi.org/10.4132/jptm.2023.11.02
Abstract
- Background
The implication of the presence of tumor-infiltrating T lymphocytes (TIL-T) in diffuse large B-cell lymphoma (DLBCL) is yet to be elucidated. We aimed to investigate the effect of TIL-T levels on the prognosis of patients with DLBCL.
Methods
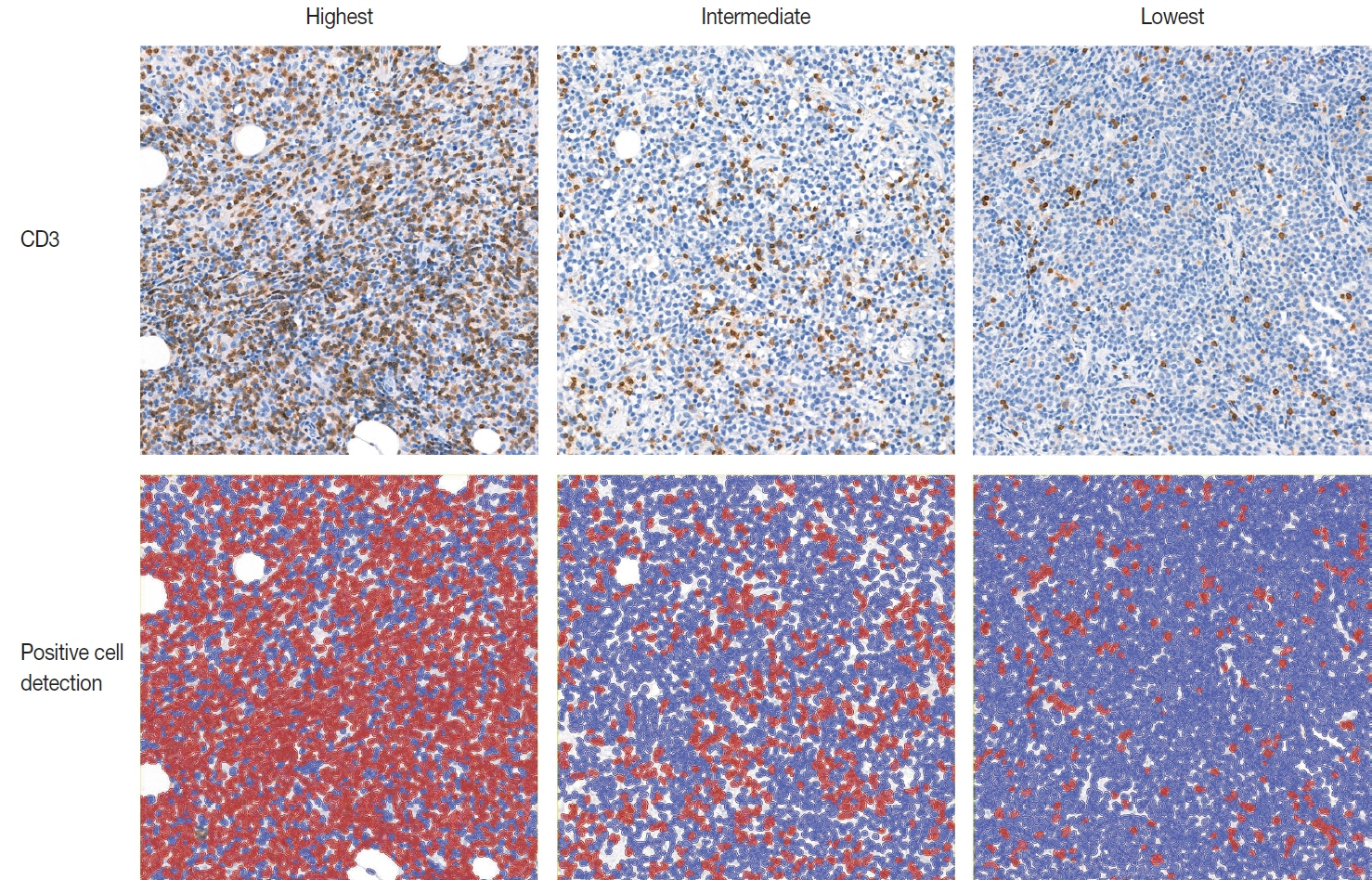
Ninety-six patients with DLBCL were enrolled in the study. The TIL-T ratio was measured using QuPath, a digital pathology software package. The TIL-T ratio was investigated in three foci (highest, intermediate, and lowest) for each case, resulting in TIL-T–Max, TIL-T–Intermediate, and TIL-T–Min. The relationship between the TIL-T ratios and prognosis was investigated.
Results
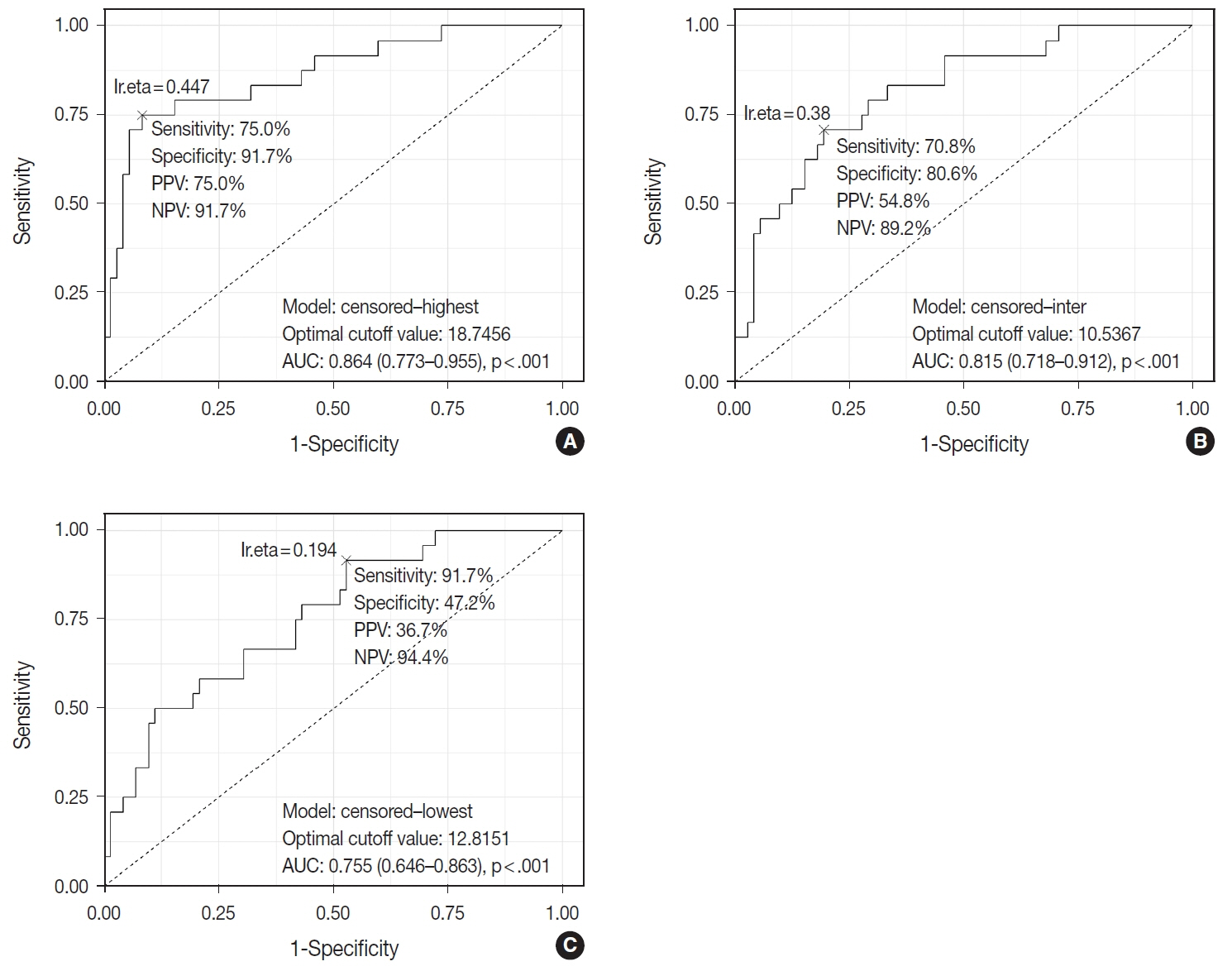
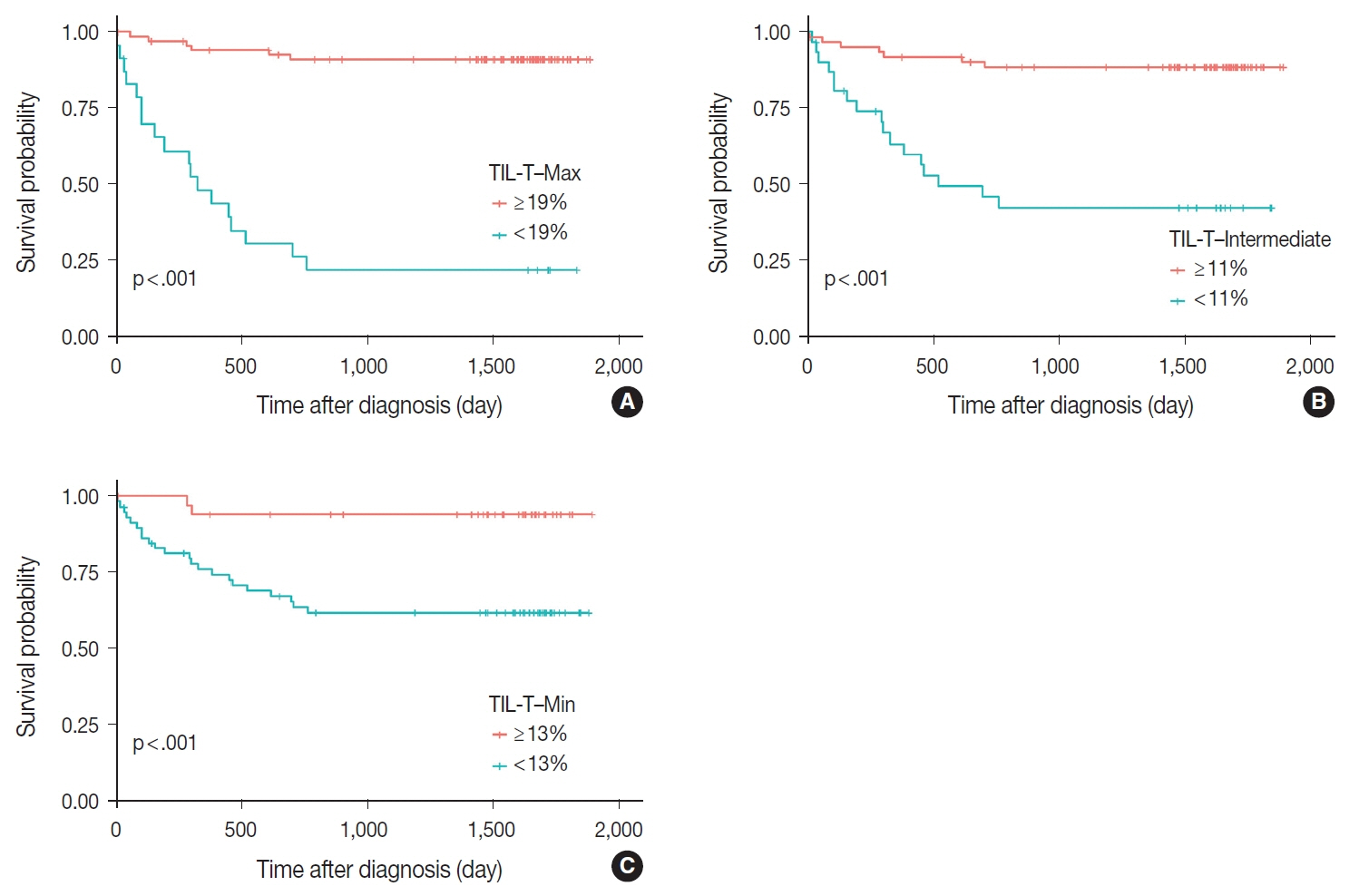
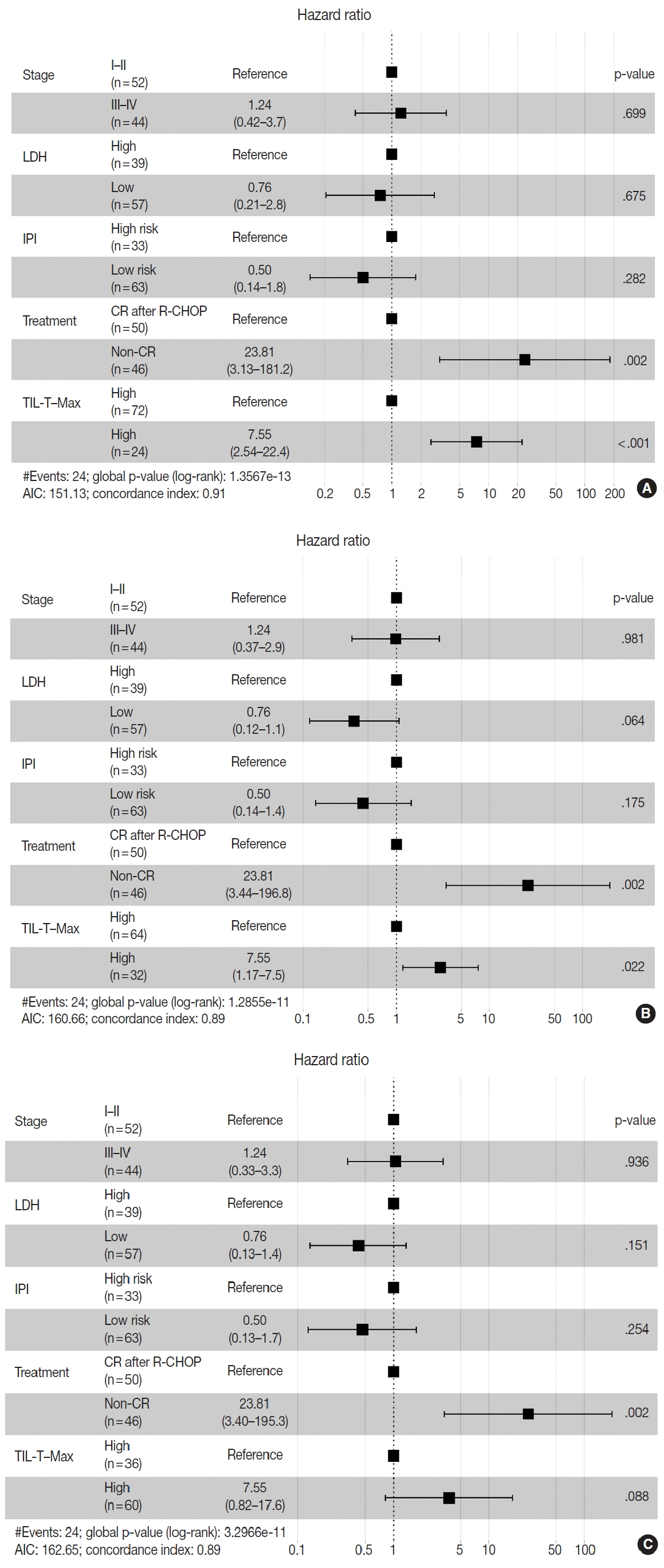
When 19% was used as the cutoff value for TIL-T–Max, 72 (75.0%) and 24 (25.0%) patients had high and low TIL-T–Max, respectively. A high TIL-T–Max was significantly associated with lower serum lactate dehydrogenase levels (p < .001), with patient group who achieved complete remission after RCHOP therapy (p < .001), and a low-risk revised International Prognostic Index score (p < .001). Univariate analysis showed that patients with a low TIL-T–Max had a significantly worse prognosis in overall survival compared to those with a high TIL-T–Max (p < .001); this difference remained significant in a multivariate analysis with Cox proportional hazards (hazard ratio, 7.55; 95% confidence interval, 2.54 to 22.42; p < .001).
Conclusions
Patients with DLBCL with a high TIL-T–Max showed significantly better prognosis than those with a low TIL-T–Max, and the TIL-T–Max was an independent indicator of overall survival. These results suggest that evaluating TIL-T ratios using a digital pathology system is useful in predicting the prognosis of patients with DLBCL.
Keyword
Figure
Reference
-
References
1. Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. 2022; 36:1720–48.2. Campo E, Jaffe ES, Cook JR, et al. The International Consensus Classification of Mature Lymphoid Neoplasms: a report from the Clinical Advisory Committee. Blood. 2022; 140:1229–53.3. Younes A, Sehn LH, Johnson P, et al. Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma. J Clin Oncol. 2019; 37:1285–95.4. Davies A, Cummin TE, Barrans S, et al. Gene-expression profiling of bortezomib added to standard chemoimmunotherapy for diffuse large B-cell lymphoma (REMoDL-B): an open-label, randomised, phase 3 trial. Lancet Oncol. 2019; 20:649–62.5. Nowakowski GS, Chiappella A, Gascoyne RD, et al. ROBUST: a phase III study of lenalidomide plus R-CHOP versus placebo plus R-CHOP in previously untreated patients with ABC-type diffuse large B-cell lymphoma. J Clin Oncol. 2021; 39:1317–28.6. Zhang J, Medeiros LJ, Young KH. Cancer immunotherapy in diffuse large B-cell lymphoma. Front Oncol. 2018; 8:351.7. Wang L, Li LR, Young KH. New agents and regimens for diffuse large B cell lymphoma. J Hematol Oncol. 2020; 13:175.8. Nicholas NS, Apollonio B, Ramsay AG. Tumor microenvironment (TME)-driven immune suppression in B cell malignancy. Biochim Biophys Acta. 2016; 1863:471–82.9. Scott DW, Gascoyne RD. The tumour microenvironment in B cell lymphomas. Nat Rev Cancer. 2014; 14:517–34.10. Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012; 12:298–306.11. Keane C, Vari F, Hertzberg M, et al. Ratios of T-cell immune effectors and checkpoint molecules as prognostic biomarkers in diffuse large B-cell lymphoma: a population-based study. Lancet Haematol. 2015; 2:e445–55.12. Xu-Monette ZY, Xiao M, Au Q, et al. Immune profiling and quantitative analysis decipher the clinical role of immune-checkpoint expression in the tumor immune microenvironment of DLBCL. Cancer Immunol Res. 2019; 7:644–57.13. Sermer D, Batlevi C, Palomba ML, et al. Outcomes in patients with DLBCL treated with commercial CAR T cells compared with alternate therapies. Blood Adv. 2020; 4:4669–78.14. Hopfinger G, Jager U, Worel N. CAR-T cell therapy in diffuse large B cell lymphoma: hype and hope. Hemasphere. 2019; 3:e185.15. Xu Y, Kroft SH, McKenna RW, Aquino DB. Prognostic significance of tumour-infiltrating T lymphocytes and T-cell subsets in de novo diffuse large B-cell lymphoma: a multiparameter flow cytometry study. Br J Haematol. 2001; 112:945–9.16. Lippman SM, Spier CM, Miller TP, Slymen DJ, Rybski JA, Grogan TM. Tumor-infiltrating T-lymphocytes in B-cell diffuse large cell lymphoma related to disease course. Mod Pathol. 1990; 3:361–7.17. Keane C, Gill D, Vari F, Cross D, Griffiths L, Gandhi M. CD4(+) tumor infiltrating lymphocytes are prognostic and independent of RIPI in patients with DLBCL receiving R-CHOP chemo-immunotherapy. Am J Hematol. 2013; 88:273–6.18. Ansell SM, Stenson M, Habermann TM, Jelinek DF, Witzig TE. Cd4+ T-cell immune response to large B-cell non-Hodgkin’s lymphoma predicts patient outcome. J Clin Oncol. 2001; 19:720–6.19. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004; 103:275–82.20. Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007; 109:1857–61.21. Bankhead P, Loughrey MB, Fernandez JA, et al. QuPath: Open source software for digital pathology image analysis. Sci Rep. 2017; 7:16878.22. Wang S, He Z, Wang X, Li H, Liu XS. Antigen presentation and tumor immunogenicity in cancer immunotherapy response prediction. Elife. 2019; 8:e4020.23. Blankenstein T, Coulie PG, Gilboa E, Jaffee EM. The determinants of tumour immunogenicity. Nat Rev Cancer. 2012; 12:307–13.24. Mukhopadhyay S, Feldman MD, Abels E, et al. Whole slide imaging versus microscopy for primary diagnosis in surgical pathology: a multicenter blinded randomized noninferiority study of 1992 cases (pivotal study). Am J Surg Pathol. 2018; 42:39–52.25. Gudlaugsson E, Skaland I, Janssen EA, et al. Comparison of the effect of different techniques for measurement of Ki67 proliferation on reproducibility and prognosis prediction accuracy in breast cancer. Histopathology. 2012; 61:1134–44.26. Stalhammar G, Robertson S, Wedlund L, et al. Digital image analysis of Ki67 in hot spots is superior to both manual Ki67 and mitotic counts in breast cancer. Histopathology. 2018; 72:974–89.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association of CD57+ Natural Killer Cells with Better Overall Survival in DLBCL Patients
- Primary Cutaneous T-cell/histiocyte-rich B-cell Lymphoma
- Primary Diffuse Large B-cell Lymphoma of the Prostate: A Case Report
- A Case of Epstein-Barr Virus-Positive Diffuse Large B-Cell Lymphoma Occurring in Thyroid Gland
- Prognostic Implication of Programmed Death-1-Positive Tumor-infiltrating Lymphocytes in Diffuse Large B-Cell Lymphoma